Abstract
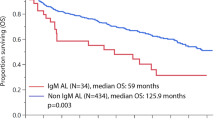
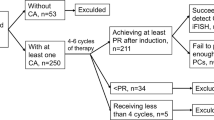
The significance of interphase fluorescence in situ hybridization (iFISH) by regimen type was assessed in 692 immunoglobulin light-chain (AL) amyloidosis patients with iFISH at diagnosis. First-line treatment was categorized as stem cell transplant and three non-transplant regimens. The most common abnormality was t(11;14) (49% of patients) followed by monosomy 13/del(13q) (36%) and trisomies (26%). A lower rate of very good partial response (VGPR) or better was observed in patients with t(11;14) treated with bortezomib-based (52% vs 77%; P=0.004) and IMiD-based regimens (13% vs 54%; P=0.04) compared with those lacking t(11;14). This corresponded to an inferior overall survival (OS) in t(11;14)-positive bortezomib-treated (median 15 vs 27 months; P=0.05) and IMiD-treated patients (median 12 vs 32 months; P=0.05). The inferior OS associated with t(11;14) bortezomib-treated patients was restricted to patients with favorable disease. Trisomies were associated with a shorter OS (median 29 vs 69 months; P=0.001), reaching statistical significance only for melphalan (median 15 vs 32 months; P=0.02). Multivariate analysis confirmed an independent survival impact for trisomies in the entire cohort and for t(11;14) among bortezomib-treated patients. iFISH is prognostic in untreated AL amyloidosis and may influence treatment selection. Patients with t(11;14) should be considered for ASCT or standard-dose melphalan at diagnosis because the survival disadvantage may be abrogated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kastritis E, Dimopoulos MA . Recent advances in the management of AL amyloidosis. Br J Haematol 2016; 172: 170–186.
Merlini G, Seldin DC, Gertz MA . Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol 2011; 29: 1924–1933.
Pardanani A, Witzig TE, Schroeder G, McElroy EA, Fonseca R, Dispenzieri A et al. Circulating peripheral blood plasma cells as a prognostic indicator in patients with primary systemic amyloidosis. Blood 2003; 101: 827–830.
Kyle RA, Linos A, Beard CM, Linke RP, Gertz MA, O’Fallon WM et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992; 79: 1817–1822.
Warsame R, Kumar SK, Gertz MA, Lacy MQ, Buadi FK, Hayman SR et al. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J 2015; 5: e310.
Abraham RS, Ballman KV, Dispenzieri A, Grill DE, Manske MK, Price-Troska TL et al. Functional gene expression analysis of clonal plasma cells identifies a unique molecular profile for light chain amyloidosis. Blood 2005; 105: 794–803.
Bochtler T, Hegenbart U, Cremer FW, Heiss C, Benner A, Hose D et al. Evaluation of the cytogenetic aberration pattern in amyloid light chain amyloidosis as compared with monoclonal gammopathy of undetermined significance reveals common pathways of karyotypic instability. Blood 2008; 111: 4700–4705.
Harrison CJ, Mazzullo H, Ross FM, Cheung KL, Gerrard G, Harewood L et al. Translocations of 14q32 and deletions of 13q14 are common chromosomal abnormalities in systemic amyloidosis. Br J Haematol 2002; 117: 427–435.
Hayman SR, Bailey RJ, Jalal SM, Ahmann GJ, Dispenzieri A, Gertz MA et al. Translocations involving the immunoglobulin heavy-chain locus are possible early genetic events in patients with primary systemic amyloidosis. Blood 2001; 98: 2266–2268.
Bryce AH, Ketterling RP, Gertz MA, Lacy M, Knudson RA, Zeldenrust S et al. Translocation t(11;14) and survival of patients with light chain (AL) amyloidosis. Haematologica 2009; 94: 380–386.
Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol 2015; 33: 1371–1378.
Bochtler T, Hegenbart U, Kunz C, Benner A, Kimmich C, Seckinger A et al. Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: a long-term follow-up study. Blood 2016; 128: 594–602.
Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia 2012; 26: 2317–2325.
Gutierrez NC, Garcia JL, Hernandez JM, Lumbreras E, Castellanos M, Rasillo A et al. Prognostic and biologic significance of chromosomal imbalances assessed by comparative genomic hybridization in multiple myeloma. Blood 2004; 104: 2661–2666.
Hu Y, Chen W, Chen S, Huang Z . Cytogenetic abnormality in patients with multiple myeloma analyzed by fluorescent in situ hybridization. Onco Targets Ther 2016; 9: 1145–1149.
Huang SY, Yao M, Tang JL, Tsay W, Lee FY, Liu MC et al. Clinical significance of cytogenetics and interphase fluorescence in situ hybridization analysis in newly diagnosed multiple myeloma in Taiwan. Ann Oncol 2005; 16: 1530–1538.
Perez-Simon JA, Garcia-Sanz R, Tabernero MD, Almeida J, Gonzalez M, Fernandez-Calvo J et al. Prognostic value of numerical chromosome aberrations in multiple myeloma: a FISH analysis of 15 different chromosomes. Blood 1998; 91: 3366–3371.
Chretien ML, Corre J, Lauwers-Cances V, Magrangeas F, Cleynen A, Yon E et al. Understanding the role of hyperdiploidy in myeloma prognosis: which trisomies really matter? Blood 2015; 126: 2713–2719.
Kumar S, Fonseca R, Ketterling RP, Dispenzieri A, Lacy MQ, Gertz MA et al. Trisomies in multiple myeloma: impact on survival in patients with high-risk cytogenetics. Blood 2012; 119: 2100–2105.
Pawlyn C, Melchor L, Murison A, Wardell CP, Brioli A, Boyle EM et al. Coexistent hyperdiploidy does not abrogate poor prognosis in myeloma with adverse cytogenetics and may precede IGH translocations. Blood 2015; 125: 831–840.
Bochtler T, Hegenbart U, Kunz C, Benner A, Seckinger A, Dietrich S et al. Gain of chromosome 1q21 is an independent adverse prognostic factor in light chain amyloidosis patients treated with melphalan/dexamethasone. Amyloid 2014; 21: 9–17.
Acknowledgements
The study was supported by the Jabbs foundation (Birmingham, UK) and the Henry J. Predolin Foundation (USA).
Author contributions
EM designed the study, analyzed the data, wrote the first draft and approved the final version of the manuscript; AD, SKK, DD, MQL, FKB, SRH, NL, WG, RW, TVK, SR, PK, JAL, YL, RSG, SZ, SVK performed patient management, revised the manuscript critically and approved the final version of the manuscript. RPK and RC provided critical review of the manuscript and approved the last version of the manuscript; RAK performed patients’ follow-up, revised the manuscript critically and participated in final data analysis and approval of the final version of the manuscript; MAG performed patient management, designed the study, analyzed the data, wrote the first draft and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AD received research funding from Celgene, Millennium, Pfizer and Janssen, and travel grant from Pfizer; SKK received consultancy and research funding from Celgene, consultancy and research funding from Millennium, research funding from Novartis, consultancy and research funding from Onyx, research funding from AbbVie, consultancy and research funding from Janssen, consultancy and research funding from BMS; DD received research funding from Karyopharm Therapeutics, Amgen and Millenium Pharmaceuticals; MQL received research funding from Celgene; PK received research funding from Takeda, Celgene and Amgen; MAG received honoraria from Gertz, Celgene, Onyx, Novartis, Smith Kline, Prothena, Ionis, Amgen and also received consultancy and honoraria from Millennium. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Muchtar, E., Dispenzieri, A., Kumar, S. et al. Interphase fluorescence in situ hybridization in untreated AL amyloidosis has an independent prognostic impact by abnormality type and treatment category. Leukemia 31, 1562–1569 (2017). https://doi.org/10.1038/leu.2016.369
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.369
This article is cited by
-
Outcomes of t(11;14) light chain (AL) amyloidosis after autologous stem cell transplantation: benchmark for new therapies
Blood Cancer Journal (2023)
-
45/w mit zunehmender Schwäche, Appetitlosigkeit, Diarrhöen und Ödemen
Der Onkologe (2022)
-
Genetic pathogenesis of immunoglobulin light chain amyloidosis: basic characteristics and clinical applications
Experimental Hematology & Oncology (2021)
-
Venetoclax induces deep hematologic remissions in t(11;14) relapsed/refractory AL amyloidosis
Blood Cancer Journal (2021)
-
Immunoglobulin light chain amyloidosis diagnosis and treatment algorithm 2021
Blood Cancer Journal (2021)