Abstract
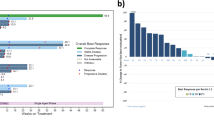
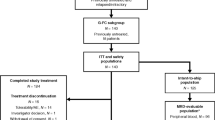
Dasatinib (DAS) and interferon-α have antileukemic and immunostimulatory effects and induce deep responses in chronic myeloid leukemia (CML). We assigned 40 newly diagnosed chronic-phase CML patients to receive DAS 100 mg o.d. followed by addition of pegylated interferon-α2b (PegIFN) after 3 months (M3). The starting dose of PegIFN was 15 μg/week and it increased to 25 μg/week at M6 until M15. The combination was well tolerated with manageable toxicity. Of the patients, 84% remained on PegIFN at M12 and 91% (DAS) and 73% (PegIFN) of assigned dose was given. Only one patient had a pleural effusion during first year, and three more during the second year. After introduction of PegIFN we observed a steep increase in response rates. Major molecular response was achieved in 10%, 57%, 84% and 89% of patients at M3, M6, M12 and M18, respectively. At M12, MR4 was achieved by 46% and MR4.5 by 27% of patients. No patients progressed to advanced phase. In conclusion, the combination treatment appeared safe with very promising efficacy. A randomized comparison of DAS±PegIFN is warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hehlmann R . CML–Where do we stand in 2015? Ann Hematol 2015; 94 (Suppl 2): S103–S105.
Hoglund M, Sandin F, Hellstrom K, Bjoreman M, Bjorkholm M, Brune M et al. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood 2013; 122: 1284–1292.
Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009; 23: 1054–1061.
Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boque C et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014; 123: 494–500.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270.
Kantarjian HM, Hochhaus A, Saglio G, De SC, Flinn IW, Stenke L et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol 2011; 12: 841–851.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016; 5: 1044–1054.
Cortes JE, Baccarani M, Guilhot F, Druker BJ, Branford S, Kim DW et al. Phase III, randomized, open-label study of daily imatinib mesylate 400mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol 2010; 28: 424–430.
Cortes JE, Kim DW, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicius L et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol 2012; 30: 3486–3492.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369: 1783–1796.
Cortes JE, Talpaz M, Kantarjian H . Ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2014; 370: 577.
Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-Study IV. J Clin Oncol 2014; 32: 415–423.
Hughes T . ENESTnd 5-year follow-up: continued benefit of frontline nilotinib vs imatinib in patients with CML in chronic phase. Haematologica 2014; 99 (suppl 1): (abstract 877).
Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010; 11: 1029–1035.
Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 2013; 122: 515–522.
Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol 2014; 32: 424–430.
Takahashi N, Kyo T, Maeda Y, Sugihara T, Usuki K, Kawaguchi T et al. Discontinuation of imatinib in Japanese patients with chronic myeloid leukemia. Haematologica 2012; 97: 903–906.
Abboud C, Berman E, Cohen A, Cortes J, DeAngelo D, Deininger M et al. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood 2013; 121: 4439–4442.
Cortes JE, Saglio G, Baccarani M, Kantarjian HM, Mayer J, Boqué C et al. Final study results of the phase 3 dasatinib versus imatinib in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) trial (DASISION, CA180-056). Blood 2014; 124: 152–152.
Hjorth-Hansen H, Stenke L, Soderlund S, Dreimane A, Ehrencrona H, Gedde-Dahl T et al. Dasatinib induces fast and deep responses in newly diagnosed chronic myeloid leukaemia patients in chronic phase: clinical results from a randomised phase-2 study (NordCML006). Eur J Haematol 2015; 94: 243–250.
Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood 2012; 119: 1123–1129.
Radich JP, Kopecky KJ, Appelbaum FR, Kamel-Reid S, Stock W, Malnassy G et al. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic-phase chronic myeloid leukemia. Blood 2012; 120: 3898–3905.
Interferon alfa versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials. Chronic Myeloid Leukemia Trialists' Collaborative Group. J Natl Cancer Inst 1997; 89: 1616–1620.
Simonsson B, Hjorth-Hansen H, Bjerrum OW, Porkka K . Interferon for treatment of chronic myeloid leukemia. Curr Drug Targets 2010; 12: 420–428.
Talpaz M, Hehlmann R, Quintas-Cardama A, Mercer J, Cortes J . Re-emergence of interferon-alpha in the treatment of chronic myeloid leukemia. Leukemia 2013; 27: 803–812.
Mahon FX, Delbrel X, Cony-Makhoul P, Faberes C, Boiron JM, Barthe C et al. Follow-up of complete cytogenetic remission in patients with chronic myeloid leukemia after cessation of interferon alfa. J Clin Oncol 2002; 20: 214–220.
Bhattacharya S, Zheng H, Tzimas C, Carroll M, Baker DP, Fuchs SY . Bcr-abl signals to desensitize chronic myeloid leukemia cells to IFNalpha via accelerating the degradation of its receptor. Blood 2011; 118: 4179–4187.
Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol 2011; 29: 1634–1642.
Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med 2010; 363: 2511–2521.
Johnson-Ansah H, Guilhot J, Rousselot P, Rea D, Legros L, Rigal-Huguet F et al. Tolerability and efficacy of pegylated interferon-α-2a in combination with imatinib for patients with chronic-phase chronic myeloid leukemia. Cancer 2013; 119: 4284–4289.
Simonsson B, Gedde-Dahl T, Markevarn B, Remes K, Stentoft J, Almqvist A et al. Combination of pegylated interferon-{alpha}2b with imatinib increases molecular response rates in patients with low or intermediate risk chronic myeloid leukemia. Blood 2011; 12: 3228–3235.
Cortes J, Quintas-Cardama A, Jones D, Ravandi F, Garcia-Manero G, Verstovsek S et al. Immune modulation of minimal residual disease in early chronic phase chronic myelogenous leukemia: a randomized trial of frontline high-dose imatinib mesylate with or without pegylated interferon alpha-2b and granulocyte-macrophage colony-stimulating factor. Cancer 2011; 117: 572–580.
Baccarani M, Martinelli G, Rosti G, Trabacchi E, Testoni N, Bassi S et al. Imatinib and pegylated human recombinant interferon-alpha2b in early chronic-phase chronic myeloid leukemia. Blood 2004; 104: 4245–4251.
Palandri F, Castagnetti F, Iacobucci I, Martinelli G, Amabile M, Gugliotta G et al. The response to imatinib and interferon-alpha is more rapid than the response to imatinib alone: a retrospective analysis of 495 Philadelphia-positive chronic myeloid leukemia patients in early chronic phase. Haematologica 2010; 95: 1415–1419.
Palandri F, Iacobucci I, Castagnetti F, Testoni N, Poerio A, Amabile M et al. Front-line treatment of Philadelphia positive chronic myeloid leukemia with imatinib and interferon-alpha: 5-year outcome. Haematologica 2008; 93: 770–774.
Nicolini FE, Etienne G, Dubruille V, Roy L, Huguet F, Legros L et al. Nilotinib and peginterferon alfa-2a for newly diagnosed chronic-phase chronic myeloid leukaemia (NiloPeg): a multicentre, non-randomised, open-label phase 2 study. Lancet Haematol 2015; 2: e37–e46.
Giles FJ, Mauro MJ, Hong F, Ortmann CE, McNeill C, Woodman RC et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia 2013; 27: 1310–1315.
Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 2010; 116: 772–782.
Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 2000; 6: 1018–1023.
Porkka K, Khoury HJ, Paquette RL, Matloub Y, Sinha R, Cortes JE . Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer 2010; 116: 377–386.
Burchert A, Wolfl S, Schmidt M, Brendel C, Denecke B, Cai D et al. Interferon-alpha, but not the ABL-kinase inhibitor imatinib (STI571), induces expression of myeloblastin and a specific T-cell response in chronic myeloid leukemia. Blood 2003; 101: 259–264.
Cross NCP, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 2015; 29: 999–1003.
Hochhaus A, Rosti G, Cross NCP, Steegmann JL, le Coutre P, Ossenkoppele G et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia 2015; 30: 57–64.
Cross NC, White HE, Muller MC, Saglio G, Hochhaus A . Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012; 26: 2172–2175.
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. 2013 Blood 2013; 122: 872–884.
Kim TD, Rea D, Schwarz M, Grille P, Nicolini FE, Rosti G et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia 2013; 27: 1316–1321.
O'Brien SG, Hedgley C, Adams S, Foroni L, Apperley JF, Holyoake TL et al. Spirit 2: an NCRI randomised study comparing dasatinib with imatinib in patients with newly diagnosed CML. Blood 2014; 124http://www.spirit-cml.org/media/99599/ash-92014-s99592-slides.pdf.
Rea D, Nicolini FE, Tulliez M, Rousselot P, Guilhot F, Gardembas M et al. Dasatinib or nilotinib discontinuation in chronic phase (CP)-chronic myeloid leukemia (CML) patients (pts) with durably undetectable BCR-ABL transcripts: interim analysis of the STOP 2G-TKI Study with a minimum follow-up of 12 months - on behalf of the French CML Group Filmc. Blood 2014; 124.
Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol 2015; 2: e528–e535.
Roy L, Chomel J-C, Guilhot J, Guerci-Bresler A, Escoffre-Barbe M, Giraudier P et al. Combination of dasatinib and Peg-interferon alpha 2b in chronic phase chronic myeloid leukemia (CP-CML) first line: preliminary results of a phase II trial, from the French Intergroup of CML (Fi-LMC). American Society of Hematology Annual meeting, Orlando, FL, USA, 2015, p. 134 Abstract.
Acknowledgements
We thank Berit Bjelkåsen, Ann Jorunn Sandstå and Sven M Carlsen at the Dept of Applied Clinical Research (AKF), NTNU for WebCRF and other assistance during this study. We thank patients and all study personnel for their efforts. The Norwegian University of Science and Technology (NTNU, Trondheim, Norway) sponsored the study on behalf of the Nordic CML Study Group (NCMLSG). The trial was supported by a grant from Bristol-Myers Squibb to NTNU. Neither sponsor nor BMS had any role in study design, collection, analysis or interpretation of data or preparation of this report.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The Nordic CML Study group is an open academic forum for research on CML, and has performed clinical studies and received financing for this in collaborations with BMS, Novartis, Pfizer and MSD. The present study was supported by MSD and BMS by provision of study drug free of charge and BMS gave financial support for study administration. Individual investigators have the following ties to declare: Henrik Hjorth-Hansen: ARIAD and BMS (consulting); Jesper Stentoft: Novartis, BMS, Pfizer, ARIAD (study support); Johan Richter: ARIAD, BMS and Novartis (consulting); Perttu Koskenvesa: ARIAD (consulting); Martin Höglund: Akinion Oharmaceuticals (consulting) and Janssen-Cilag (temporary advisory board); Kimmo Porkka: Novartis, BMS and Pfizer honoraria (advisory board or speaker) and research funding; Bjørn Tore Gjertsen: ARIAD, Bayer, BerGenBio, Pfizer, MedImmune, Novartis and Roche (consulting and advisory boards). Stock equity owner: KinN Therapeutics and ACT2, Ole Weis Bjerrum: BMS, Novartis, Ariad and Pfizer; Satu Mustjoki: Novartis, BMS and Pfizer honoraria (advisory board or speaker) and Ariad research funding; Ulla Olsson-Strömberg: ARIAD (honoraria). The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Hjorth-Hansen, H., Stentoft, J., Richter, J. et al. Safety and efficacy of the combination of pegylated interferon-α2b and dasatinib in newly diagnosed chronic-phase chronic myeloid leukemia patients. Leukemia 30, 1853–1860 (2016). https://doi.org/10.1038/leu.2016.121
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.121
This article is cited by
-
Developing therapeutic approaches for chronic myeloid leukemia: a review
Molecular and Cellular Biochemistry (2023)
-
Switching from imatinib to nilotinib plus pegylated interferon-α2b in chronic phase CML failing to achieve deep molecular response: clinical and immunological effects
Annals of Hematology (2023)
-
Direct and indirect effects of IFN-α2b in malignancy treatment: not only an archer but also an arrow
Biomarker Research (2022)
-
Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape
Leukemia (2021)
-
Tyrosine Kinase Inhibitors and Beyond for Chronic Myeloid Leukemia in Children
Pediatric Drugs (2021)