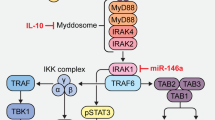
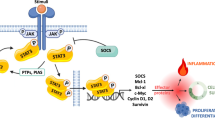
Abstract
The Philadelphia-negative myeloproliferative neoplasms (MPNs) are clonal disorders involving hematopoietic stem and progenitor cells and are associated with myeloproliferation, splenomegaly and constitutional symptoms. Similar signs and symptoms can also be found in patients with chronic inflammatory diseases, and inflammatory processes have been found to play an important role in the pathogenesis and progression of MPNs. Signal transduction pathways involving JAK1, JAK2, STAT3 and STAT5 are causally involved in driving both the malignant cells and the inflammatory process. Moreover, anti-inflammatory and immune-modulating drugs have been used successfully in the treatment of MPNs. However, to date, many unresoved issues remain. These include the role of somatic mutations that are present in addition to JAK2V617F, CALR and MPL W515 mutations, the interdependency of malignant and nonmalignant cells and the means to eradicate MPN-initiating and -maintaining cells. It is imperative for successful therapeutic approaches to define whether the malignant clone or the inflammatory cells or both should be targeted. The present review will cover three aspects of the role of inflammation in MPNs: inflammatory states as important differential diagnoses in cases of suspected MPN (that is, in the absence of a clonal marker), the role of inflammation in MPN pathogenesis and progression and the use of anti-inflammatory drugs for MPNs. The findings emphasize the need to separate the inflammatory processes from the malignancy in order to improve our understanding of the pathogenesis, diagnosis and treatment of patients with Philadelphia-negative MPNs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Milosevic JD, Kralovics R . Genetic and epigenetic alterations of myeloproliferative disorders. Int J Hematol 2013; 97: 183–197.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013; 369: 2379–2390.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013; 369: 2391–2405.
Cabagnols X, Favale F, Pasquier F, Messaoudi K, Defour JP, Ianotto JC et al. Presence of atypical thrombopoietin receptor (MPL) mutations in triple negative essential thrombocythemia patients. Blood 2015; 127: 333–342.
Milosevic Feenstra JD, Nivarthi H, Gisslinger H, Leroy E, Rumi E, Chachoua I et al. Whole exome sequencing identifies novel MPL and JAK2 mutations in triple negative myeloproliferative neoplasms. Blood 2015; 127: 325–332.
Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martínez-Trillos A, Casetti I et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood 2014; 124: 1062–1069.
Rotunno G, Mannarelli C, Guglielmelli P, Pacilli A, Pancrazzi A, Pieri L et al. Impact of calreticulin mutations on clinical and hematological phenotype and outcome in essential thrombocythemia. Blood 2014; 123: 1552–1555.
Jaffe ES, Harris NL, Vardiman J, Campo E, Arber DM . Hematopathology. Elsevier Health Sciences 2010.
Swerdlow SH, Campo E, Harris NL, Jaffe E, Pileri S, Stein H et al. WHO Classification of Tumors of the Haematopoietic and Lymphoid Tissues. IARC: Lyon, France, 2008.
Buhr T, Hebeda K, Kaloutsi V, Porwit A, Van der Walt J, Kreipe H . European Bone Marrow Working Group trial on reproducibility of World Health Organization criteria to discriminate essential thrombocythemia from prefibrotic primary myelofibrosis. Haematologica 2012; 97: 360–365.
Lu M, Xia L, Liu YC, Hochman T, Bizzari L, Aruch D et al. Lipocalin produced by myelofibrosis cells affects the fate of both hematopoietic and marrow microenvironmental cells. Blood 2015; 126: 972–982.
Fleischman AG, Aichberger KJ, Luty SB, Bumm TG, Petersen CL, Doratotaj S et al. TNFα facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood 2011; 118: 6392–6398.
Kleppe M, Kwak M, Koppikar P, Riester M, Keller M, Bastian L et al. JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov 2015; 5: 316–331.
Reynaud D, Pietras E, Barry-Holson K, Mir A, Binnewies M, Jeanne M et al. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell 2011; 20: 661–673.
Welner RS, Amabile G, Bararia D, Czibere A, Yang H, Zhang H et al. Treatment of chronic myelogenous leukemia by blocking cytokine alterations found in normal stem and progenitor cells. Cancer Cell 2015; 27: 671–681.
Tefferi A . Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol 2005; 23: 8520–8530.
Hoermann G, Cerny-Reiterer S, Herrmann H, Blatt K, Bilban M, Gisslinger H et al. Identification of oncostatin M as a JAK2 V617F-dependent amplifier of cytokine production and bone marrow remodeling in myeloproliferative neoplasms. FASEB J 2012; 26: 894–906.
Le Bousse-Kerdiles MC, Chevillard S, Charpentier A, Romquin N, Clay D, Smadja-Joffe F et al. Differential expression of transforming growth factor-beta, basic fibroblast growth factor, and their receptors in CD34+ hematopoietic progenitor cells from patients with myelofibrosis and myeloid metaplasia. Blood 1996; 88: 4534–4546.
Martyre MC, Le Bousse-Kerdiles MC, Romquin N, Chevillard S, Praloran V, Demory JL et al. Elevated levels of basic fibroblast growth factor in megakaryocytes and platelets from patients with idiopathic myelofibrosis. Bri J Haematol 1997; 97: 441–448.
Bock O, Hoftmann J, Theophile K, Hussein K, Wiese B, Schlue J et al. Bone morphogenetic proteins are overexpressed in the bone marrow of primary myelofibrosis and are apparently induced by fibrogenic cytokines. Am J Pathol 2008; 172: 951–960.
Verstovsek S, Mesa RA, Foltz LM, Gupta V, Mascarenhas JO, Ritchie EK et al. Phase 2 trial of PRM-151, an anti-fibrotic agent, in patients with myelofibrosis: stage 1 results. Blood 2014; 124: 713–713.
Panteli KE, Hatzimichael EC, Bouranta PK, Katsaraki A, Seferiadis K, Stebbing J et al. Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol 2005; 130: 709–715.
Estrov Z, Kurzrock R, Wetzler M, Kantarjian H, Blake M, Harris D et al. Suppression of chronic myelogenous leukemia colony growth by interleukin-1 (IL-1) receptor antagonist and soluble IL-1 receptors: a novel application for inhibitors of IL-1 activity. Blood 1991; 78: 1476–1484.
Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A . Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol 2011; 29: 1356–1363.
Barbui T, Carobbio A, Finazzi G, Guglielmelli P, Salmoiraghi S, Rosti V et al. Elevated C-reactive protein is associated with shortened leukemia-free survival in patients with myelofibrosis. Leukemia 2013; 27: 2084–2086.
Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med 2010; 363: 1117–1127.
Kroger N, Holler E, Kobbe G, Bornhauser M, Schwerdtfeger R, Baurmann H et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood 2009; 114: 5264–5270.
Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica 2011; 96: 315–318.
Falanga A, Marchetti M, Evangelista V, Vignoli A, Licini M, Balicco M et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood 2000; 96: 4261–4266.
Villmow T, Kemkes-Matthes B, Matzdorff AC . Markers of platelet activation and platelet-leukocyte interaction in patients with myeloproliferative syndromes. Thromb Res 2002; 108: 139–145.
Falanga A, Marchetti M, Vignoli A, Balducci D, Barbui T . Leukocyte-platelet interaction in patients with essential thrombocythemia and polycythemia vera. Exp Hematol 2005; 33: 523–530.
Tam CS, Nussenzveig RM, Popat U, Bueso-Ramos CE, Thomas DA, Cortes JA et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood 2008; 112: 1628–1637.
Hasselbalch HC . Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 2012; 119: 3219–3225.
Catassi C, Bearzi I, Holmes GK . Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology 2005; 128: S79–S86.
Lukas M . Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis 2010; 28: 619–624.
Hasselbalch HC . A role of NF-E2 in chronic inflammation and clonal evolution in essential thrombocythemia, polycythemia vera and myelofibrosis? Leuk Res 2014; 38: 263–266.
Hasselbalch HC . The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev 2013; 24: 133–145.
Hasselbalch HC . Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk Res 2013; 37: 214–220.
Kaufmann KB, Grunder A, Hadlich T, Wehrle J, Gothwal M, Bogeska R et al. A novel murine model of myeloproliferative disorders generated by overexpression of the transcription factor NF-E2. J Exp Med 2012; 209: 35–50.
Goerttler PS, Kreutz C, Donauer J, Faller D, Maiwald T, Marz E et al. Gene expression profiling in polycythaemia vera: overexpression of transcription factor NF-E2. Br J Haematol 2005; 129: 138–150.
Wang W, Schwemmers S, Hexner EO, Pahl HL . AML1 is overexpressed in patients with myeloproliferative neoplasms and mediates JAK2V617F-independent overexpression of NF-E2. Blood 2010; 116: 254–266.
Jutzi JS, Bogeska R, Nikoloski G, Schmid CA, Seeger TS, Stegelmann F et al. MPN patients harbor recurrent truncating mutations in transcription factor NF-E2. J Exp Med 2013; 210: 1003–1019.
Jutzi JS, Pahl HL . The hen or the egg: inflammatory aspects of murine MPN models. Mediators Inflamm 2015; 2015: 101987.
Wehrle J, Seeger TS, Schwemmers S, Pfeifer D, Bulashevska A, Pahl HL . Transcription factor nuclear factor erythroid-2 mediates expression of the cytokine interleukin 8, a known predictor of inferior outcome in patients with myeloproliferative neoplasms. Haematologica 2013; 98: 1073–1080.
Roelz R, Pilz IH, Mutschler M, Pahl HL . Of mice and men: human RNA polymerase III promoter U6 is more efficient than its murine homologue for shRNA expression from a lentiviral vector in both human and murine progenitor cells. Exp Hematol 2010; 38: 792–797.
Zhang Y, Liu X, McHale C, Li R, Zhang L, Wu Y et al. Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. PLoS One 2013; 8: e74974.
Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 2015; 525: 389–393.
Sorensen AL, Hasselbalch HC . Smoking and Philadelphia-negative chronic myeloproliferative neoplasms. Eur J Haematol 2015; e-pub ahead of print 18 September 2015 doi:10.1111/ejh.12684.
Silver RT, Kiladjian JJ, Hasselbalch HC . Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev Hematol 2013; 6: 49–58.
Utke Rank C, Weis Bjerrum O, Larsen TS, Kjær L, de Stricker K, Riley CH et al. Minimal residual disease after long-term interferon-alpha2 treatment. A report on hematological, molecular, and histomorphological response patterns in ten patients with essential thrombocythemia and polycythemia vera. Leuk Lymph 2015; e-pub ahead of print 18 June 2015.
Deininger M, Radich J, Burn TC, Huber R, Paranagama D, Verstovsek S . The effect of long-term ruxolitinib treatment on JAK2p.V617F allele burden in patients with myelofibrosis. Blood 2015; 126: 1551–1554.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: 799–807.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: 787–798.
Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 2015; 372: 426–435.
Meyer SC, Keller MD, Chiu S, Koppikar P, Guryanova OA, Rapaport F et al. CHZ868, a type II JAK2 inhibitor, reverses type I JAK inhibitor persistence and demonstrates efficacy in myeloproliferative neoplasms. Cancer Cell 2015; 28: 15–28.
Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R et al. Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood 2014; 123: 2075–2083.
Mascarenhas J . Rationale for combination therapy in myelofibrosis. Best Pract Res Clin Haematol 2014; 27: 197–208.
Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell 1998; 93: 373–383.
Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW . Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 2002; 285: 1–24.
Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol 2011; 29: 789–796.
Mascarenhas J, Talpaz M, Gupta V, Savona M, Coughlin P, Winton E et al. An open-label, phase II study of the JAK1 inhibitor INCB039110 in patients with myelofibrosis. Blood 2013; 122: 663–663.
Kiladjian J-J, Mesa RA, Hoffman R . The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011; 117: 4706–4715.
Silver RT, Vandris K, Goldman JJ . Recombinant interferon-alpha may retard progression of early primary myelofibrosis: a preliminary report. Blood 2011; 117: 6669–6672.
Pizzi M, Silver RT, Barel A, Orazi A . Recombinant interferon-alpha in myelofibrosis reduces bone marrow fibrosis, improves its morphology and is associated with clinical response. Mod Pathol 2015; 28: 1315–1323.
Hasan S, Lacout C, Marty C, Cuingnet M, Solary E, Vainchenker W et al. JAK2V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNα. Blood 2013; 122: 1464–1477.
Mullally A, Bruedigam C, Poveromo L, Heidel FH, Purdon A, Vu T et al. Depletion of Jak2V617F myeloproliferative neoplasm-propagating stem cells by interferon-α in a murine model of polycythemia vera. Blood 2013; 121: 3692–3702.
Lu M, Zhang W, Li Y, Berenzon D, Wang X, Wang J et al. Interferon-alpha targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp Hematol 2010; 38: 472–480.
Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol 2011; 29: 761–770.
Hasselbalch HC . Perspectives on the impact of JAK-inhibitor therapy upon inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Expert Rev Hematol 2014; 7: 203–216.
Bjorn ME, de Stricker K, Kjaer L, Ellemann K, Hasselbalch HC . Combination therapy with interferon and JAK1-2 inhibitor is feasible: proof of concept with rapid reduction in JAK2V617F-allele burden in polycythemia vera. Leuk Res Rep 2014; 3: 73–75.
Acknowledgements
This study summarizes the results of discussions and deliberations during a series of interactive working sessions organized by the European LeukemiaNet MPN group between June 2014 and February 2015. We thank Professors Radek Skoda, Sylvie Hermouet, Vladan Čokić, Dominik Wolf, Hans-Michael Kvasnicka, Jerry Spivak and Martin Griesshammer for participating in some of the ELN MPN group workshops and for their helpful suggestions, and the Alpine Oncology Foundation (AOF) team, in particular Dr Alpa Parmar, for arranging the workshops. AOF acknowledges receiving unrestricted educational research support from Novartis Oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Steffen Koschmieder: research funding (Novartis and Novartis Foundation); consultancy and advisory boards (Ariad, AOP, Baxalta, Bristol-Myers Squibb, CTI, Novartis, Pfizer, Sanofi); honoraria and travel grants (Ariad, Alexion, AOP, Baxalta, Bristol-Myers Squibb, Celgene, CTI, Novartis, Pfizer, Sanofi, Shire). Hans C Hasselbalch: research grant (Novartis). Peter Valent: honoraria (Novartis, Ariad, Pfizer und BMS); grant support (Novartis, Ariad); consultancy (Novartis Global PKC412 Trial). Heike Pahl: consultancy and advisory boards (AOP, Baxalta, Novartis, Shire). Rüdiger Hehlmann: research funding (BMS and Novartis). Alessandro Vannucchi: honoraria for advisory board and speaker fees (Novartis). Francisco Cervantes: advisory board (Novartis, AOP, and CTI-Baxter); speakers bureau (Novartis and CTI-Baxter).
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Koschmieder, S., Mughal, T., Hasselbalch, H. et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia 30, 1018–1024 (2016). https://doi.org/10.1038/leu.2016.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.12
This article is cited by
-
Inherited polygenic effects on common hematological traits influence clonal selection on JAK2V617F and the development of myeloproliferative neoplasms
Nature Genetics (2024)
-
Validation of myeloproliferative neoplasms associated risk factor RDW as predictor of thromboembolic complications in healthy individuals: analysis on 6849 participants of the SHIP-study
Leukemia (2023)
-
Primäre Myelofibrose
Die Onkologie (2023)
-
Interleukin-1 contributes to clonal expansion and progression of bone marrow fibrosis in JAK2V617F-induced myeloproliferative neoplasm
Nature Communications (2022)
-
DUSP6 mediates resistance to JAK2 inhibition and drives leukemic progression
Nature Cancer (2022)