Abstract
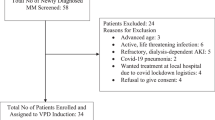
We aimed at demonstrating non-inferiority of bortezomib/cyclophosphamide/dexamethasone (VCD) compared to bortezomib/doxorubicin/dexamethasone (PAd) induction therapy with respect to very good partial response rates or better (⩾VGPR) in 504 newly diagnosed, transplant-eligible multiple myeloma patients. VCD was found to be non-inferior to PAd with respect to ⩾VGPR rates (37.0 versus 34.3%, P=0.001). The rates of progressive disease (PD) were 0.4% (VCD) versus 4.8% (PAd; P=0.003). In the PAd arm, 11 of 12 patients with PD had either renal impairment (creatinine ⩾2 mg/dl) at diagnosis or the cytogenetic abnormality gain 1q21, whereas no PD was observed in these subgroups in the VCD arm. Leukocytopenia/neutropenia (⩾3°) occurred more frequently in the VCD arm (35.2% versus 11.3%, P<0.001). Neuropathy rates (⩾2°) were higher in the PAd group (14.9 versus 8.4%, P=0.03). Serious adverse events, both overall and those related to thromboembolic events, were higher in the PAd group (32.7 versus 24.0%, P=0.04 and 2.8 versus 0.4%, P=0.04). Stem cell collection was not impeded by VCD. VCD is as effective as PAd in terms of achieving ⩾VGPR rates with fewer PD and has a favorable toxicity profile. Therefore, VCD is preferable to PAd as induction therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Palumbo A, Anderson K . Multiple myeloma. N Engl J Med 2011; 364: 1046–1060.
Engelhardt M, Terpos E, Kleber M, Gay F, Wasch R, Morgan G et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica 2014; 99: 232–242.
Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, Blade J et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood 2011; 117: 6063–6073.
Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood 2012; 120: 1589–1596.
Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012; 119: 4375–4382.
Palumbo A, Gay F, Falco P, Crippa C, Montefusco V, Patriarca F et al. Bortezomib as induction before autologous transplantation, followed by lenalidomide as consolidation-maintenance in untreated multiple myeloma patients. J Clin Oncol 2010; 28: 800–807.
Sonneveld P, Schmidt-Wolf IGH, van der Holt B, El Jarari L, Bertsch U, Salwender H et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol 2012; 30: 2946–2955.
Harousseau J-L, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol 2010; 28: 4621–4629.
Sonneveld P, Goldschmidt H, Rosinol L, Blade J, Lahuerta JJ, Cavo M et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol 2013; 31: 3279–3287.
Moreau P, Attal M, Pegourie B, Planche L, Hulin C, Facon T et al. Achievement of VGPR to induction therapy is an important prognostic factor for longer PFS in the IFM 2005-01 trial. Blood 2011; 117: 3041–3044.
O’Shea D, Giles C, Terpos E, Perz J, Politou M, Sana V et al. Predictive factors for survival in myeloma patients who undergo autologous stem cell transplantation: a single-centre experience in 211 patients. Bone Marrow Transplant 2006; 37: 731–737.
Alvares CL, Davies FE, Horton C, Patel G, Powles R, Sirohi B et al. Long-term outcomes of previously untreated myeloma patients: responses to induction chemotherapy and high-dose melphalan incorporated within a risk stratification model can help to direct the use of novel treatments. Br J Haematol 2005; 129: 607–614.
Khan ML, Reeder CB, Kumar SK, Lacy MQ, Reece DE, Dispenzieri A et al. A comparison of lenalidomide/dexamethasone versus cyclophosphamide/lenalidomide/dexamethasone versus cyclophosphamide/bortezomib/dexamethasone in newly diagnosed multiple myeloma. Br J Haematol 2012; 156: 326–333.
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 2009; 23: 1337–1341.
Davies FE, Wu P, Jenner M, Srikanth M, Saso R, Morgan GJ . The combination of cyclophosphamide, velcade and dexamethasone (CVD) induces high response rates with comparable toxicity to velcade alone (V) and velcade plus dexamethasone (VD). Haematologica 2007; 92: 1149–1150.
Einsele H, Liebisch P, Langer C, Kropff M, Wandt H, Jung W et al. Velcade, intravenous cyclophosphamide and dexamethasone (VCD) induction for previously untreated multiple myeloma (German DSMM XIa Trial). Blood 2009; 114: 59a–60a, Abstract # 131.
Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet 2010; 376: 2075–2085.
Kyle RA, Rajkumar SV . Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2008; 23: 3–9.
Durie BGM, Harousseau J-L, Miguel JS, Blade J, Barlogie B, Anderson K et al. International uniform response criteria for multiple myeloma. Leukemia 2006; 20: 1467–1473.
Greipp PR, San Miguel J, Durie BGM, Crowley JJ, Barlogie B, Blade J et al. International staging system for multiple myeloma. J Clin Oncol 2005; 23: 3412–3420.
Blade J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G et alMyeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol 1998; 102: 1115–1123.
Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood 2012; 119: 940–948.
Newcombe RG . Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med 1998; 17: 873–890.
Farrington CP, Manning G . Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat Med 1990; 9: 1447–1454.
Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 1999; 341: 1565–1571.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2013; 28: 1122–1128.
Chanan-Khan AA, Giralt S . Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol 2010; 28: 2612–2624.
Harousseau J-L, Attal M, Avet-Loiseau H . The role of complete response in multiple myeloma. Blood 2009; 114: 3139–3146.
Gay F, Larocca A, Wijermans P, Cavallo F, Rossi D, Schaafsma R et al. Complete response correlates with long-term progression-free and overall survival in elderly myeloma treated with novel agents: analysis of 1175 patients. Blood 2011; 117: 3025–3031.
Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol 2013; 31: 4529–4535.
San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008; 359: 906–917.
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Laumann K et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood 2010; 115: 3416–3417.
Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010; 116: 679–686.
Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the intergroupe Francophone du Myelome. J Clin Oncol 2014; 32: 2712–2717.
Moreau P, Avet-Loiseau H, Facon T, Attal M, Tiab M, Hulin C et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood 2011; 118: 5752–5758.
Ludwig H, Viterbo L, Greil R, Masszi T, Spicka I, Shpilberg O et al. Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. J Clin Oncol 2013; 31: 247–255.
Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood 2012; 120: 1801–1809.
Reeder CB, Reece DE, Kukreti V, Mikhael JR, Chen C, Trudel S et al. Long-term survival with cyclophosphamide, bortezomib and dexamethasone induction therapy in patients with newly diagnosed multiple myeloma. Br J Haematol 2014; 167: 563–565.
Zhang J, Su Y-M, Li D, Cui Y, Huang Z-Z, Wei J-Y et al. TNF-α-mediated JNK activation in the dorsal root ganglion neurons contributes to bortezomib-induced peripheral neuropathy. Brain Behav Immun 2014; 38: 185–191.
Ravaglia S, Corso A, Piccolo G, Lozza A, Alfonsi E, Mangiacavalli S et al. Immune-mediated neuropathies in myeloma patients treated with bortezomib. Clin Neurophysiol 2008; 119: 2507–2512.
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 2011; 12: 431–440.
Merz M, Salwender H-J, Hanel M, Bertsch U, Kunz C, Hielscher T et al. Subcutaneous versus intravenous bortezomib in two different induction therapies for newly diagnosed multiple myeloma – subgroup analysis from the GMMG-MM5 trial. Blood 2014; 124: 3475.
Brioli A, Zannetti BA, Zamagni E, Tacchetti P, Pantani L, Mancuso K et al. Peripheral neuropathy induced by subcutaneous bortezomib‐based induction therapy for newly diagnosed multiple myeloma. Haematologica 2014; 99: e242–e243.
Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau J-L et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol 2009; 144: 895–903.
Zangari M, Siegel E, Barlogie B, Anaissie E, Saghafifar F, Fassas A et al. Thrombogenic activity of doxorubicin in myeloma patients receiving thalidomide: implications for therapy. Blood 2002; 100: 1168–1171.
Hassoun H, Reich L, Klimek VM, Dhodapkar M, Cohen A, Kewalramani T et al. Doxorubicin and dexamethasone followed by thalidomide and dexamethasone is an effective well tolerated initial therapy for multiple myeloma. Br J Haematol 2006; 132: 155–161.
Acknowledgements
We thank the investigators, the study nurses and all the members of the study teams at the participating GMMG trial sites, the teams of the myeloma research laboratory, the FISH laboratory and the central laboratory at the University Hospital Heidelberg, the coordination centers for clinical trials (KKS) in Heidelberg and Leipzig, the pharmacies at the trial sites and, most importantly, the participating patients and their families. This study was supported by grants from Celgene, Janssen-Cilag, Chugai and Binding Site.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
EKM received travel grants from Janssen-Cilag, Celgene, Onyx and Mundipharma. JD received honoraria from Janssen-Cilag, Celgene, Roche and GSK, and compensation for his consulting/advisory roles for Janssen-Cilag, Roche and GSK. MMu received honoraria from Celgene as well as travel grants from Mundipharma and Celgene. DH received compensation for his consulting/advisory role from Celgene and also received research funding from Novartis and Celgene. JH received honoraria from Celgene and Janssen-Cilag. IGHS-W received compensation for his consulting/advisroy role from Janssen-Cilag. KW received honoraria from Celgene, Janssen-Cilag and Onyx; received compensation for her consulting/advisory role at Celgene, Janssen, Onyx and Noxxon; and also received research funding from Celgene and travel grants from Celgene and Janssen-Cilag. CS received honoraria and compensation for his consulting/advisory role from Janssen-Cilag and Celgene. HS received honoraria from Celgene, Janssen-Cilag, Chugai, Novartis, Mundipharma, TEVA, Roche, Binding Site and BMS; received compensation for his consulting/advisory role at Celgene, Janssen-Cilag, BMS, Novartis and TEVA; received research funding from Celgene, Janssen-Cilag, Novartis and BMS; and also received travel grants from Celgene, Janssen-Cilag, Novartis, TEVA and Roche. HG received honoraria from Janssen-Cilag, Celgene, Novartis, Onyx and Millennium; has a consulting/advisory role at Janssen-Cilag, Celgene, Novartis, Onyx and Millennium; received compensation as a member of the speakers bureau from Janssen-Cilag, Celgene, Novartis, Onyx, Millennium and Chugai; and also received research funding from Janssen-Cilag, Celgene, Novartis and Chugai. UB, CK, MH, IWB, AJ, BS, TH, MMe, BH-D, AS, MSR, KN, H-WL, MZ and CG declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Mai, E., Bertsch, U., Dürig, J. et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia 29, 1721–1729 (2015). https://doi.org/10.1038/leu.2015.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.80
This article is cited by
-
Predictors of early morbidity and mortality in newly diagnosed multiple myeloma: data from five randomized, controlled, phase III trials in 3700 patients
Leukemia (2023)
-
Impact of the changing landscape of induction therapy prior to autologous stem cell transplantation in 540 newly diagnosed myeloma patients: a retrospective real-world study
Journal of Cancer Research and Clinical Oncology (2023)
-
What’s Old is New: The Past, Present and Future Role of Thalidomide in the Modern-Day Management of Multiple Myeloma
Targeted Oncology (2022)
-
Weekly cyclophosphamide, subcutaneous bortezomib and dexamethathasone (CyBorD) for initial treatment of transplant-eligible patients with multiple myeloma: experience of two transplant centres
Bone Marrow Transplantation (2021)
-
Intensives Therapiekonzept mit innovativen Substanzen bei älteren, fitten Patienten
InFo Hämatologie + Onkologie (2021)