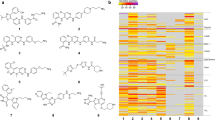
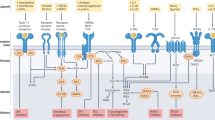
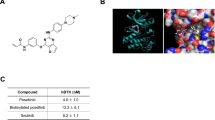
Abstract
Proteomic-based drug testing is an emerging approach to establish the clinical value and anti-neoplastic potential of multikinase inhibitors. The multikinase inhibitor midostaurin (PKC412) is a promising new agent used to treat patients with advanced systemic mastocytosis (SM). We examined the target interaction profiles and the mast cell (MC)-targeting effects of two pharmacologically relevant midostaurin metabolites, CGP52421 and CGP62221. All three compounds, midostaurin and the two metabolites, suppressed IgE-dependent histamine secretion in basophils and MC with reasonable IC50 values. Midostaurin and CGP62221 also produced growth inhibition and dephosphorylation of KIT in the MC leukemia cell line HMC-1.2, whereas the second metabolite, CGP52421, which accumulates in vivo, showed no substantial effects. Chemical proteomic profiling and drug competition experiments revealed that midostaurin interacts with KIT and several additional kinase targets. The key downstream regulator FES was recognized by midostaurin and CGP62221, but not by CGP52421 in MC lysates, whereas the IgE receptor downstream target SYK was recognized by both metabolites. Together, our data show that the clinically relevant midostaurin metabolite CGP52421 inhibits IgE-dependent histamine release, but is a weak inhibitor of MC proliferation, which may have clinical implications and may explain why mediator-related symptoms improve in SM patients even when disease progression occurs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Valent P, Akin C, Sperr WR, Horny HP, Arock M, Lechner K et al. Diagnosis and treatment of systemic mastocytosis: state of the art. Br J Haematol 2003; 122: 695–717.
Akin C, Metcalfe DD . Systemic mastocytosis. Annu Rev Med 2004; 55: 419–432.
Arock M, Valent P . Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol 2010; 3: 497–516.
Horny HP, Valent P . Diagnosis of mastocytosis: general histopathological aspects, morphological criteria, and immunohistochemical findings. Leuk Res 2001; 25: 543–551.
Horny HP, Sotlar K, Valent P . Mastocytosis: state of the art. Pathobiology 2007; 74: 121–132.
Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA 1995; 92: 10560–10564.
Longley BJ, Tyrrell L, Lu SZ, Ma YS, Langley K, Ding TG et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet 1996; 12: 312–314.
Longley BJ, Metcalfe DD, Tharp M, Wang X, Tyrrell L, Lu SZ et al. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc Natl Acad Sci USA 1999; 96: 1609–1614.
Fritsche-Polanz R, Jordan JH, Feix A, Sperr WR, Sunder-Plassmann G, Valent P et al. Mutation analysis of C-KIT in patients with myelodysplastic syndromes without mastocytosis and cases of systemic mastocytosis. Br J Haematol 2001; 113: 357–364.
Féger F, Ribadeau Dumas A, Leriche L, Valent P, Arock M . Kit and c-kit mutations in mastocytosis: a short overview with special reference to novel molecular and diagnostic concepts. Int Arch Allergy Immunol 2002; 127: 110–114.
Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res 2003; 27: 635–641.
Escribano L, Akin C, Castells M, Orfao A, Metcalfe DD . Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol 2002; 81: 677–690.
Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest 2007; 37: 435–453.
Brockow K, Jofer C, Behrendt H, Ring J . Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy 2008; 63: 226–232.
González-de-Olano D, Alvarez-Twose I, Vega A, Orfao A, Escribano L . Venom immunotherapy in patients with mastocytosis and hymenoptera venom anaphylaxis. Immunotherapy 2011; 3: 637–651.
Carter MC, Robyn JA, Bressler PB, Walker JC, Shapiro GG, Metcalfe DD . Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol 2007; 119: 1550–1551.
Valent P, Sperr WR, Schwartz LB, Horny HP . Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol 2004; 114: 3–11.
Tefferi A, Verstovsek S, Pardanani A . How we diagnose and treat WHO-defined systemic mastocytosis in adults. Haematologica 2008; 93: 6–9.
Valent P, Sperr WR, Akin C . How I treat patients with advanced systemic mastocytosis. Blood 2010; 116: 5812–5817.
Gotlib J, Berubé C, Growney JD, Chen CC, George TI, Williams C et al. Activity of the tyrosine kinase inhibitor PKC412 in a patient with mast cell leukemia with the D816V KIT mutation. Blood 2005; 106: 2865–2870.
Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Böhm A et al. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood 2006; 107: 752–759.
Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C . Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood 2006; 108: 286–291.
Gleixner KV, Mayerhofer M, Sonneck K, Gruze A, Samorapoompichit P, Baumgartner C et al. Synergistic growth-inhibitory effects of two tyrosine kinase inhibitors, dasatinib and PKC412, on neoplastic mast cells expressing the D816V-mutated oncogenic variant of KIT. Haematologica 2007; 92: 1451–1459.
Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O et al. KIT inhibitor midostaurin in patients with advanced systemic mastocytosis: results of a planned interim analysis of the global CPKC412D2201 trial. Blood 2012; 120: 799.
Ustun C, DeRemer DL, Akin C . Tyrosine kinase inhibitors in the treatment of systemic mastocytosis. Leuk Res 2011; 35: 1143–1152.
Akin C, Brockow K, D'Ambrosio C, Kirshenbaum AS, Ma Y, Longley BJ et al. Effects of tyrosine kinase inhibitor STI571 on human mast cells bearing wild-type or mutated c-kit. Exp Hematol 2003; 31: 686–692.
Fabbro D, Ruetz S, Bodis S, Pruschy M, Csermak K, Man A et al. PKC412 - a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des 2000; 15: 17–28.
Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD, Gilliland DG . Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood 2005; 106: 721–724.
Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 2008; 26: 127–132.
Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O et al. Durable responses and improved quality of life with midostaurin (PKC412) in advanced systemic mastocytosis (SM): updated stage 1 results of the global D2201 trial. Blood 2013; 122: 106.
Kneidinger M, Schmidt U, Rix U, Gleixner KV, Vales A, Baumgartner C et al. The effects of dasatinib on IgE receptor-dependent activation and histamine release in human basophils. Blood 2008; 111: 3097–3107.
Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P . Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy 2009; 39: 1711–1720.
Propper DJ, McDonald AC, Man A, Thavasu P, Balkwill F, Braybrooke JP et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J. Clin Oncol 2001; 19: 1485–1492.
Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105: 54–60.
Levis M, Brown P, Smith BD, Stine A, Pham R, Stone R et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood 2006; 108: 3477–3483.
Dutreix C, Munarini F, Lorenzo S, Roesel J, Wang Y . Investigation into CYP3A4-mediated drug-drug interactions on midostaurin in healthy volunteers. Cancer Chemother Pharmacol 2013; 72: 1223–1234.
Wang Y, Yin OQ, Graf P, Kisicki JC, Schran H . Dose- and time-dependent pharmacokinetics of midostaurin in patients with diabetes mellitus. J Clin Pharmacol 2008; 48: 763–775.
Valent P, Ashman LK, Hinterberger W, Eckersberger F, Majdic O, Lechner K et al. Mast cell typing: demonstration of a distinct hematopoietic cell type and evidence for immunophenotypic relationship to mononuclear phagocytes. Blood 1989; 73: 1778–1785.
Peter B, Cerny-Reiterer S, Hadzijusufovic E, Schuch K, Stefanzl G, Eisenwort G et al. The pan-Bcl-2 blocker obatoclax promotes the expression of Puma, Noxa, and Bim mRNA and induces apoptosis in neoplastic mast cells. J Leukoc Biol 2014; 95: 95–104.
Butterfield JH, Weiler D, Dewald G, Gleich GJ . Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res 1988; 12: 345–355.
Rix U, Hantschel O, Dürnberger G, Remsing Rix LL, Planyavsky M, Fernbach NV et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood 2007; 110: 4055–4063.
Hantschel O, Rix U, Schmidt U, Bürckstümmer T, Kneidinger M, Schütze G et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc Natl Acad Sci USA 2007; 104: 13283–13288.
Fernbach NV, Planyavsky M, Müller A, Breitwieser FP, Colinge J, Rix U et al. Acid elution and one-dimensional shotgun analysis on an Orbitrap mass spectrometer: an application to drug affinity chromatography. J Proteome Res 2009; 8: 4753–4765.
Bennett KL, Funk M, Tschernutter M, Breitwieser FP, Planyavsky M, Ubaida Mohien C et al. Proteomic analysis of human cataract aqueous humour: comparison of one-dimensional gel LCMS with two-dimensional LCMS of unlabelled and iTRAQ(R)-labelled specimens. J Proteomics 2011; 74: 151–166.
Hoermann G, Cerny-Reiterer S, Perné A, Klauser M, Hoetzenecker K, Klein K et al. Identification of oncostatin M as a STAT5-dependent mediator of bone marrow remodeling in KIT D816V-positive systemic mastocytosis. Am J Pathol 2011; 178: 2344–2356.
Böhm A, Sonneck K, Gleixner KV, Schuch K, Pickl WF, Blatt K et al. In vitro and in vivo growth-inhibitory effects of cladribine on neoplastic mast cells exhibiting the imatinib-resistant KIT mutation D816V. Exp Hematol 2010; 38: 744–755.
Voisset E, Lopez S, Dubreuil P, De Sepulveda P . The tyrosine kinase FES is an essential effector of KITD816V proliferation signal. Blood 2007; 110: 2593–2599.
Kluin-Nelemans HC, Oldhoff JM, Van Doormaal JJ, Van 't Wout JW, Verhoef G, Gerrits WB et al. Cladribine therapy for systemic mastocytosis. Blood 2003; 102: 4270–4276.
Acknowledgements
This study was supported by the Austrian Science Fund (FWF): F 4704-B20, F 4711-B20, F 4611-B19, and P 21173-B13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PV is a consultant in a global midostaurin trial sponsored by Novartis and received grant support and honoraria from Novartis. AR is a consultant in a global midostaurin trial sponsored by Novartis and received honoraria from Novartis. CD, JR and PWM are employed by Novartis Pharma AG. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Peter, B., Winter, G., Blatt, K. et al. Target interaction profiling of midostaurin and its metabolites in neoplastic mast cells predicts distinct effects on activation and growth. Leukemia 30, 464–472 (2016). https://doi.org/10.1038/leu.2015.242
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.242