Abstract
Despite the success of imatinib mesylate (IM) in the early chronic phase of chronic myeloid leukemia (CML), patients are resistant to IM and other kinase inhibitors in the later stages of CML. Our findings indicate that inhibition of Janus kinase 2 (Jak2) in Bcr–Abl+ cells overcomes IM resistance although the precise mechanism of Jak2 action is unknown. Knocking down Jak2 in Bcr–Abl+ cells reduced levels of the Bcr–Abl protein and also the phosphorylation of Tyr177 of Bcr–Abl, and Jak2 overexpression rescued these knockdown effects. Treatment of Bcr–Abl+ cells with Jak2 inhibitors for 4–6 h but not with IM also reduced Bcr–Abl protein and pTyr177 levels. In vitro kinase experiments performed with recombinant Jak2 showed that Jak2 readily phosphorylated Tyr177 of Bcr–Abl (a Jak2 consensus site, YvnV) whereas c-Abl did not. Importantly, Jak2 inhibition decreased pTyr177 Bcr–Abl in immune complexes but did not reduce levels of Bcr–Abl, suggesting that the reduction of Bcr–Abl by Jak2 inhibition is a separate event from phosphorylation of Tyr177. Jak2 inhibition by chemical inhibitors (TG101209/WP1193) and Jak2 knockdown diminished the activation of Ras, PI-3 kinase pathways and reduced levels of pTyrSTAT5. These findings suggest that Bcr–Abl stability and oncogenic signaling in CML cells are under the control of Jak2.
Similar content being viewed by others
Introduction
Our previous experiments suggest that Janus kinase 2 (Jak2) has an important role in Bcr–Abl+ cells, and that Bcr–Abl expression leads to activation of Jak2.1, 2, 3 Bcr–Abl is known to drive the Grb2-Ras-Raf-Mek1/2 Erk pathway and the PI-3 kinase pathway involving Gab2,4, 5, 6 the Jak2-STAT3 pathway7, 8 and the Bcr–Abl-STAT5 pathway.9, 10 Phosphorylation of Tyr 177 Bcr–Abl is a critical event required for development of chronic myeloid leukemia (CML), as the Y177F mutant of Bcr–Abl diminishes CML disease.4, 11, 12 pTyr177 binds Grb2 leading to Grb2–SOS complex formation and activation of the Ras pathway. Continuous treatment with IM induces IM resistance because of a number of events including mutations in the tyrosine kinase domain (for example, T315I),13 amplification of the Bcr–Abl gene14, 15 and a Bcr–Abl independent mechanism involving Lyn kinase (for example, K562-R cells).16, 17 All these events lead to poor responses to IM therapy, allowing progression of the disease. Jak2 activation in Bcr–Abl+ cells appears not to involve phosphorylation of Jak2 by the Bcr–Abl tyrosine kinase but the interaction of Bcr–Abl with the IL-3 receptor chains, specifically the β-chain of the receptor.18, 19 The Bcr–Abl/Jak2 signaling pathway appears to be housed in a high-molecular weight structure called the Bcr–Abl network complex.20, 21, 22 Importantly, Jak2 kinase inhibition overcomes IM resistance by inducing apoptosis in IM-resistant cell lines (including T315I cells) and also cells from CML patients at the blast crisis stage.20, 21
Residual CML disease appears to involve primitive progenitor cells, which have been shown to be present in niches in the bone marrow.23, 24 These cells are considered quiescent and not Bcr–Abl dependent.25, 26, 27 These findings require a search for new therapeutic targets and compounds to eradicate these tyrosine kinase inhibitors-resistant cells from the bone marrow niche.
Jak2 is an important signaling component in hematopoietic cells, as it transmits signals generated by interaction of cytokines such as IL-3 with the IL-3 receptor. The α- and β-chains of the IL-3 receptor are part of a large dodecamer structure in which two Jak2 molecules are bound to the β-chain in close proximity to each other.28 Jak2, as a result of interaction of IL-3 with the IL-3 receptor, becomes activated by autophosphorylation at Tyr 1007.28, 29 Studies by Huang et al.30 suggest that IL-3 signaling driven by activated Jak2 is enhanced by Jak1 interaction with Jak2. Our new findings indicate that Jak2 controls Bcr–Abl signaling in CML cells, as either Jak2 knockdown or Jak2 inhibition drastically reduced the levels of Bcr–Abl and the phosphorylation of Tyr 177 within Bcr–Abl causing reduction of oncogenic signaling.
Materials and methods
Results
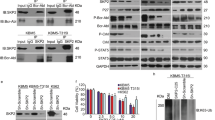
Jak2 knockdown reduced levels of Bcr–Abl protein and Tyr 177 phosphorylated form of Bcr–Abl in mouse hematopoietic and CML cell lines
Our findings with Bcr–Abl+ cell lines including cells expressing imatinib mesylate (IM)-resistant forms of Bcr–Abl indicate that Jak2 is a critical player in CML.20, 22 We began exploring the mechanisms behind this critical role of Jak2 in CML. We found that Jak2 controls Bcr–Abl protein levels, as Jak2 knockdown causes a rapid disappearance of Bcr–Abl (Figure 1). We used a specific mouse form of Jak2 small interfering RNA to knockdown Jak2 in Bcr–Abl+ 32D mouse myeloid cells (Figure 1a). Jak2 knockdown dramatically reduced levels of the pTyr Bcr–Abl protein. To offset the possible nonspecific effects of Jak2 knockdown, we rescued Jak2 knockdown by transducing Jak2 cDNA into cells at the time of Jak2 knockdown. The rescued cells had restored levels of pTyr Bcr–Abl in cells corresponding to the restored expression of Jak2, suggesting that Jak2 controls the expression of Bcr–Abl.
Knockdown of Jak2 strongly reduced levels of the Bcr–Abl protein and pTyr 177 of Bcr–Abl in Bcr–Abl+ cells and CML cell lines. (a) Knockdown of Jak2 and rescue of Jak2 expression in Bcr–Abl+ 32D cells (32Dp210) using Jak2-specific small interfering RNA. 32Dp210 contains a b3a2 form of Bcr–Abl expressed in mouse myeloid 32D cells. (b) Knockdown of Jak2 in K562-R CML cell line by an inducible Jak2-specific short hairpin RNA. Jak2 knockdown was initiated by addition of doxicycline (2 μg/ml) for 3 days. (c) Non-targeted short hairpin RNA has no effects on Jak2 expression and Bcr–Abl expression. (d) Reversal of Jak2 knockdown restores levels of pTyr177 and the Bcr–Abl protein. After a 3-day induction, doxicycline was withdrawn from the culture for 6 days. Western blotting with the appropriate antibodies was performed in these studies.
We also used an inducible form of specific human Jak2 short hairpin RNA to knockdown Jak2 in three CML cell lines, namely BV173, KBM-7 and K562-R. Levels of Jak2 were reduced and also Bcr–Abl protein levels were drastically reduced following Jak2 knockdown in K562-R cells (Figures 1b and c). Reversal of Jak2 knockdown (removal of the inducer doxicycline) restored the expression of the Bcr–Abl protein (Figure 1d).
In Jak2 knockdown experiments, levels of Tyr177 phosphorylation within Bcr–Abl were also strongly inhibited as expected as Bcr–Abl had also disappeared. Importantly, rescue experiments partially restored levels of pTyr177 (Figures 1a–d). Similar results were observed by Jak2 knockdown in CML cell lines BV173 and KBM7 (Supplementary Figure 1a–c).
Jak2 phosphorylated the tyrosine 177 Bcr sequence found in Bcr–Abl
An analysis of the Bcr–Abl sequence revealed various tyrosine residues that are Jak2 consensus phosphorylating sites (YxxV/L/I) (Supplementary Table 1).31 We observed that tyrosine 177 within Bcr–Abl (YxxV) fits the consensus Jak2 phosphorylation motif. To test whether Jak2 would phosphorylate Tyr177, we made a Bcr peptide that contains sequences surrounding the tyrosine 177 sequence of Bcr–Abl, and used that peptide as a target for Jak2 (Figures 2a–c). Purified recombinant Jak2 (JH1 domain) readily phosphorylated this peptide and this phosphorylation was strongly inhibited by the selective Jak2 inhibitor TG101209 (TG) but not by IM (Figures 2a and b). We note that TG is a potent inhibitor of Jak2's ability to phosphorylate Tyr 177 in kinase assays with an ∼IC 50 of less than 0.01 μM (reported to be 6 nM)35 (Supplementary Figure 1d).
Jak2 phosphorylates Bcr–Abl on Tyr177. (a) Jak2 inhibitor TG101209 (TG, TargeGen Inc., San Diego, CA, USA) but not IM inhibited phosphorylation of a Bcr Tyr177 peptide (custom synthesized by Bachem Co., Torrance, CA, USA) in Jak2 immune complexes from the cell lysates of 32Dp210 cells immunoprecipitated with anti-Jak2 antibody coupled to agarose beads. The target peptide has the Bcr sequence H-Ala-Glu-Lys-Pro-Phe-Tyr(177)-Val-Asn-Val-Glu-Phe-His-His-Glu-(Lys-Lys-Lys). The bolded amino acids are the Grb2 binding site in Bcr–Abl (YVNV) and are the Jak2 consensus phosphorylation site. The three Lys residues allow binding to Whatman 3 mm filters. Anti-Jak2 immune complexes were incubated in a kinase assay with γ p32 ATP in the presence of various concentrations of either Jak2 inhibitor TG or IM. (b) Jak2 inhibition of recombinant Jak2 (JH1 domain, 50 ng; Invitrogen, Carlsbad, CA, USA) by TG but not IM inhibited phosphorylation of a Tyr177 Bcr peptide. The Jak2 kinase assay with γ p32 ATP was performed using recombinant Jak2 kinase (JH1 domain, GST-Jak2 aa808–1132 in presence of either Jak2 inhibitor TG or IM using the Bcr synthetic target peptide containing Tyr177 sequence. (c) Jak2 efficiently phosphorylates the Tyr177 Bcr whereas c-Abl prefers to phosphorylate the YxxP sequence. Recombinant JH1 of Jak2 was used to phosphorylate either the Bcr Tyr177 peptide or the Abltide peptide (BIOMOL International, Plymouth Meeting, PA, USA). (d) Jak2 inhibition reduced levels of pTyr177 Bcr–Abl in kinase assays performed with anti-Jak2 immune complexes but did not reduce levels of Bcr–Abl. Mouse 32Dp210 cells were lysed and anti-Jak2 immune complexes were harvested as described.2 Lysates were western blotted with either pTyr 177 Bcr antibody or anti-Abl 8e9 antibody. (e) Jak2 inhibition reduced levels of pTyr 177 Bcr–Abl in kinase assays performed with anti-Abl p6D monoclonal antibody2 but had no effect on levels of the Bcr–Abl protein.
Jak2 immune complexes isolated from Bcr–Abl+ 32D cells also phosphorylated the Tyr 177 Bcr peptide and phosphorylation was inhibited by TG but not by IM (Figure 2a). These Jak2 immune complexes contain Bcr–Abl, Jak2 and HSP90 and other signaling members such as Akt and STAT3.20, 21 Thus, although Bcr–Abl is present in the Jak2 immune complex (see Supplementary Figure 1a, b), it does not phosphorylate Tyr 177 as IM does not inhibit tyrosine phosphorylation of the peptide (Figure 2a). We note that purified near full-length recombinant c-Abl kinase only poorly phosphorylated the Tyr 177 site in the Bcr peptide (Figure 2c).
Jak2 inhibition of phosphorylation of Tyr177 is a separate event from disappearance of Bcr–Abl
To determine whether the disappearance of Bcr–Abl could be separated from the inhibition of Tyr 177 phosphorylation, we performed kinase assays with immune complexes harvested from Bcr–Abl+ 32D cells with anti-Jak2 antibodies, and determined whether Jak2 inhibition would decrease levels of pTyr177 and whether levels of Bcr–Abl in the immune complexes would also be reduced by Jak2 inhibition. The Bcr–Abl protein was not decreased in these immune complexes by Jak2 inhibition but importantly levels of pTyr177 were strongly decreased (Figures 2d, e). Similarly, levels of pTyr Bcr–Abl were not reduced by treatment of the kinase reaction mixture with 5 and 10 μM TG (Figure 2d). Importantly, Jak2 and Bcr–Abl coprecipitated in immune complexes (Supplementary Figure 1e and f). These results indicate that the events leading to the decrease of Bcr–Abl occurred within intact cells but not in immune complexes from these same cells and, more importantly, the inhibition of phosphorylation of Tyr177 by TG can readily occur in these subcellular fractions under conditions wherein Bcr–Abl levels were stable. Importantly, we found that Jak2 inhibition caused only background levels of apoptosis during the first 4 h of treatment wherein Bcr–Abl levels were strongly reduced (Supplementary Figure 5c).
Jak2 inhibitor TG101209 rapidly decreased the levels of pTyr177 Bcr–Abl
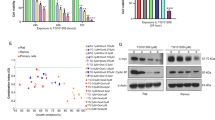
We tested the effects of Jak2 inhibition in various Bcr–Abl+ cell lines and CML cell lines. Jak2 inhibition reduced levels of active Jak2 in a dose-dependent manner.22 The 50% point of Jak2 inhibition as measured by pTyr1007 phosphorylation was estimated to be 5 μM as estimated by quantitation of the intensity values (Figure 3a). Similarly, the level of Bcr–Abl pTyr177 was inhibited in a dose-dependent manner and the 50% inhibitory point was estimated to be 5 μM. The 50% inhibitory point for Bcr–Abl reduction was estimated to be ∼7.5 μM. Interestingly, the selective Jak2 inhibitor TG rapidly decreased levels of phosphorylation of Tyr177 of Bcr–Abl but also decreased levels of Bcr–Abl during this time period (Figure 3b). The CML cell line K562-R showed rapid loss of pTyr177 Bcr–Abl and the Bcr–Abl protein after 3 h (Figure 3c), as did KBM7 CML cells (Supplementary Figure 1g). Reductions of pTyr177 Bcr–Abl and Bcr–Abl were seen in CML cell line K562 (Supplementary Figure 1h). Cells from a blast crisis CML patient treated with TG101209 also had a reduction of pTyr177 Bcr–Abl and Bcr–Abl protein (Figure 3d). We also showed that Jak2 inhibition rapidly reduced the levels of total pTyr proteins (Figure 3e).
Jak2 inhibition by TG101209 induced dephosphorylation of Bcr–Abl Tyr177 and reduction of Bcr–Abl. (a) Bcr–Abl pTyr177 and Bcr–Abl levels were reduced in 32Dp210 cells treated with indicated doses of Jak2 inhibitor TG for 16 h. Anti-Abl immunoprecipitates (IP) (P6D monoclonal antibody, custom made) were probed by western blotting (WB) with sequence-specific Bcr pTyr177 antibody (Novus Biologicals, Littleton, CO, USA) and anti-Abl 8E9. Anti-actin (Sigma Chem Co., St Louis, MO, USA) blots were performed on the supernatant before IP as a loading control. IQ represents normalized intensity performed by estimating the band intensity and dividing that by the intensity of the actin band. (b) Jak2 inhibition by TG rapidly reduced pTyr177 Bcr–Abl but had little effect on the level of Bcr–Abl levels. 32Dp210 cells were treated with the 10 μM TG for various times up to 2 h and assayed by western blotting. (c) Jak2 inhibition by TG in CML cell line K562-R reduced levels of Bcr–Abl pTyr177 and Bcr–Abl protein. (d) Cells from a blast crisis (78% blasts) CML patient showed reduced levels of pTyr177 Bcr–Abl and Bcr–Abl by Jak2 inhibition. Monocytes were isolated by Histopaque (Sigma Chem Co.) separation and grown for 24 h in culture medium without growth factors before TG treatment. (e) Jak2 inhibition by TG rapidly reduced levels of pTyr-containing proteins. Lysates were blotted with 4G10 anti-pTyr antibody. (f) CD34+ progenitor cells from a blast crisis CML patient (98% blasts) showed rapid reduction of pTyr177 Bcr–Abl and Bcr–Abl upon treatment with TG for up to 2 h. After isolation of monocytes as in panel d, cells were then fractionated on CD34 antibody magnetic beads. An aliquot assayed by flow cytometry showed that the sample was 87%+ for CD34+ cells. The CD34+ cells were treated with 10 μM TG for up to 2 h in cell culture. (g) Jak2 inhibition by TG reduced levels of pTyr177 Bcr–Abl, Bcr–Abl, Jak2, pTyr1007 Jak2 in human cord blood CD34+ progenitor cells transduced with BCR–ABL as described.12 TG treatment was done for 6 h. (h) Treatment of 32Dp210 cells with IM had little effect on levels of pTyr177 Bcr–Abl and Bcr–Abl protein levels over a 3-h period.
The Jak2 consensus sites include YxxV/L/I31 and there are a number of these Jak2 consensus sites in Bcr–Abl (Supplementary Table 1). We wondered whether YxxF would also be a site that is phosphorylated by Jak2, as F-like V/L/I is a hydrophobic amino acid. There are three such sites in Bcr–Abl including Tyr 360. We had made a mouse monoclonal antibody against the pTyr360 sequence of Bcr. This sequence-specific pTyr monoclonal antibody detected a signal in Bcr–Abl, and Jak2 inhibition by a new Jak2 inhibitor WP1193 (see below) dramatically reduced the level of pTyr360 Bcr–Abl within 60 min (Supplementary Figure 2a), suggesting that Jak2 also phosphorylates Tyr360 of Bcr–Abl.
pTyr177 within P160 BCR is also rapidly reduced by Jak2 inhibition
Similar to Bcr–Abl, phosphorylation of Tyr177 within P160 BCR was also rapidly reduced by treatment with 10 μM TG (Supplementary Figure 1i). As Bcr is believed to form heterotetramers with Bcr–Abl, these results suggest that Jak2 would phosphorylate Tyr177 within Bcr–Abl and Bcr in these heterotetramers. It is unknown whether phosphorylation of Bcr on Tyr177 would contribute to activation of Ras and PI-3 kinase pathways in CML cells.
CML 34+ cells respond to Jak2 inhibition by reduction of pTyr177 Bcr–Abl and Bcr–Abl
Cells were harvested from the peripheral blood of a CML blast crisis patient, having 98% blasts. After CD34 selection, sufficient cells were available for western blotting (3 million cells). Flow cytometry results indicated that 87% of the cells bound to the CD34 beads were CD34+ (results not shown). Western blotting revealed that Jak2 inhibition by TG reduced levels of pTyr177 and Bcr–Abl in CD34+ cells (Figure 3f). We note that CD34+cells from this patient were resistant to IM but sensitive to TG (Supplementary Figure 4a).
Cord blood CD34+ cells showed loss of Bcr–Abl and pTyr177 Bcr–Abl caused by Jak2 inhibition
We examined the effects of Jak2 inhibition in CD34+ cord blood cells transduced with BCR–ABL.12 The cells were treated for 6 h with 2.5 and 10 μM TG. pTyr177 Bcr–Abl and Bcr–Abl were drastically reduced as was Jak2 and activated Jak2 (pTyr 1007) following treatment of CD34+ cells with TG (Figure 3g). These results with CD34+ cord blood cells, which were recently transduced with Bcr–Abl, would be consistent with Jak2 inhibiting early progenitor cells in CML patients.
Inhibition of the Bcr–Abl kinase by IM had little effect on the phosphorylation of Tyr177 of Bcr–Abl in short-term experiments
Importantly, IM, a well-known Abl kinase inhibitor, did not inhibit tyrosine phosphorylation of Tyr177 of Bcr–Abl for up to 3 h within Bcr–Abl+ cells nor was the level of the Bcr–Abl protein affected during 3-h treatment with IM (Figure 3h). These results provide further evidence that Tyr177 of Bcr–Abl is a site phosphorylated by Jak2 and does not result from a Bcr–Abl autophosphorylation reaction. However, treatment of Bcr–Abl+ cells with IM for longer times, as expected, drastically decreased Bcr–Abl protein levels in a dose-dependent manner (1–10 μM, 16 h, not shown).
Jak2 inhibition reduced activation of the Ras, PI-3 kinase and STAT5 pathways
We determined whether binding of Grb2 to Bcr–Abl was similarly decreased by Jak2 inhibition. It is known that Grb2 binds to pTyr177 of Bcr–Abl and pTyr Shc; Shc also contains the Grb2-binding sequence YvnV sequence. Shc associates with Bcr–Abl and like pTyr177, drives the Ras and PI-3 kinase pathways. Grb2 levels in the Bcr–Abl immunocomplex was reduced by ∼60% within 120 min of TG treatment compared with the control (Figure 4a). Jak2 inhibition by TG decreased levels of RAS GTP within 60 min in 32Dp210 cells (Figures 4b and c), which is consistent with the observed decrease in Grb2 binding to Bcr–Abl. We also determined whether Jak2 inhibition decreased PI-3 kinase activation. It is known that pTyr177 binds Grb2 which in turn binds Gab2.6 Our previous studies20 indicate that Jak2 activation leads to phosphorylation of Tyr 452 of Gab2, which is involved in binding to the regulatory subunit of the PI-3 kinase leading to its activation.6 In our current studies, pTyr 452 Gab2 was rapidly decreased in Bcr–Abl+ cells after treatment with TG (Figure 4d). We note that PI-3 kinase activation as measured by a commercial kit was also decreased within 60–120 min of Jak2 inhibition (not shown). These results indicate that Jak2 inhibition rapidly inhibited both Ras and PI-3 kinase activation.
Jak2 inhibition by TG reduced activation of the Ras pathway and PI-3 kinase pathways. (a) Jak2 inhibition of 32Dp210 cells reduced binding of Grb2 to anti-Abl immunoprecipitates. (b) Jak2 inhibition by TG reduced levels of Ras GTP in 32Dp210 cells. These assays were performed with a commercial kit as described by the manufacturer. It involves IP/westerns with their RAS GTP antibody. The kit allows for generation of internal positive and negative controls; the third control is untreated Bcr–Abl+ cells. (c) Quantitation of RAS GTP levels following treatment of 32Dp210 cells with 10 μM TG. The intensity values of the RAS GTP bands were divided by the intensity of the actin band. (d) Jak2 inhibition by TG rapidly reduced levels of pTyr (452 YxxM) Gab2 in 32Dp210 cells. pTyr 452 is believed to bind to regulatory subunit of the PI-3 kinase, which is required for activation of PI-3 kinase. (e) Jak2 inhibition by TG rapidly decreased tyrosine phosphorylation of STAT5 (residue 694). (f) Knockdown of Jak2 reduced pathways related to the Bcr–Abl/Jak2 pathway in CML cell line K562-R. Jak2-specific short hairpin RNA was induced by treatment of cells with doxicycline as in Figure 1. The lysate was western blotted with the antibodies listed.
We determined whether STAT5 activation would also be downregulated by this more potent Jak2 inhibitor. Jak2 inhibition rapidly decreased pTyr levels of STAT5 (Figure 4e), which is consistent with downregulation of STAT5 transcriptional activity. It remains to be determined whether inactivation of Bcr–Abl by Jak2 interferes with Bcr–Abl's ability to activate STAT5.
Jak2 knockdown reduced levels of tyrosine phosphorylation of Shc and Gab2
The reduction of Grb2 binding to Bcr–Abl and the resultant decrease in Ras activation prompted an examination of Jak2 effects on phosphorylation of Shc. It is known that pTyr Shc (at the YvnV sequence) also binds Grb2 leading to Ras activation. Knockdown of Jak2 strongly decreased levels of pTyr Shc in CML cell line K562-R (Figure 4f) and BV173 cells (not shown). Thus, Jak2 inhibition not only reduced levels of Grb2 binding to Bcr–Abl, but Jak2 knockdown also reduced the alternate pathway for activating Ras, namely the tyrosine phosphorylation of Shc. Both of the effects of Jak2 inhibition would lead to reduced levels of Ras activation.
Jak2 knockdown also reduced levels of pTyr Gab2, which binds to Grb2,6 thereby reducing the activation of the PI-3 kinase pathway in K562-R cells. The levels of pMEK1 and 2, pSer 9 of GSK3 and pTyr STAT5 were also reduced by Jak2 knockdown (Figure 4f) in BV173 cells (not shown). Jak2 controls the Gab2/PI-3 kinase, Akt and GSK3 through its ability to induce phosphorylation of Gab220 and would control Ras activation and downstream MEK activation by Jak2's ability to phosphorylate Tyr177 of Bcr–Abl and Tyr 239/240 of Shc. We note that Jak2 knockdown did not decrease either Lyn kinase or total Grb2 levels (Figure 4f). Our findings indicate that Lyn kinase is not part of the Bcr–Abl/Jak2/HSP90 network complex.22 Thus, prolonged Jak2 inhibition would seriously depress levels of activated Ras and PI-3 kinase activation in Bcr–Abl+ leukemia cells.
A new pan Jak kinase inhibitor, inhibits Jak2 effects on Bcr–Abl and Tyr177 phosphorylation
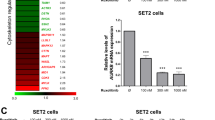
We tested the effects of a more potent analog of the Jak2 inhibitor AG490 (WP1193, Figure 5a) for its ability to reduce Bcr–Abl and to inhibit phosphorylation of Tyr177 of Bcr–Abl. WP1193 inhibited the phosphorylation of Tyr177 housed within the Bcr peptide with 50% inhibition point of <2.5 μM (Figure 5b). Like AG490, WP1193 inhibited tyrosine phosphorylation of Jak2 in cells (Figure 5c) and also inhibited tyrosine phosphorylation of Jak1 and Jak3 (not shown); WP1193 also inhibited the autophosphorylation of Jak2 in vitro (Supplementary Figure 2b). It has been reported that Jak1 kinase interacts with Jak2 leading to the strengthening of the downstream effects of cytokine signaling through Jak2.30 WP1193 rapidly reduced levels of Bcr–Abl and pTyr177 Bcr–Abl within several Bcr–Abl+ cell lines including T315I cells and cells from blast crisis CML patients (Figures 5c–e). WP1193 appeared to be more potent than TG (compare Figures 5c–e with Figures 3b–d). The estimated point of 50% inhibition of phosphorylation of Tyr177, and Bcr–Abl reduction for WP1193 was between 2.0 and 3.0 μM in whole cells, respectively (Supplementary Figure 2h). Overall, the pan Jak inhibitor, although much less potent in Jak2 kinase assays than TG101209 (estimated 50% inhibition point of about 2 μM for WP1193 compared with 0.01 μM for TG (compare Figure 5b with Supplementary Figure 1d), WP1193 was similar if not more potent at reducing levels of Bcr–Abl and pTyr177 compared with TG101209 (compare inhibition by WP1193 and TG101209 in Figures 5c–e and Figures 3b–d, respectively).
A new Jak2 inhibitor WP1193 rapidly reduced levels of pTyr177 Bcr–Abl, Bcr–Abl protein and levels of pTyr Bcr–Abl in 32Dp210 cells. (a) Structure of WP1193 and AG490. AG490 is a known Jak kinase inhibitor. (b) Jak2 inhibitor WP1193 inhibits the ability of recombinant Jak2 (JH1-JH2) to phosphorylate Tyr177 Bcr peptide. The same methods were used as described in Figure 2. (c) Top panel: Jak2 inhibitor WP1193 rapidly inhibited phosphorylation of Tyr177 of Bcr–Abl and reduced levels of Bcr–Abl in 32Dp210 cells. 32Dp210 cells were lysed and immunoprecipitated with anti-Abl p6D monoclonal antibody as described in Figure 2a. Bottom panel: 32Dp210 cells were treated for up to 1 h with 10 μM WP1193, lysates were made and immunoprecipitated with anti-Jak2 antibody. Western blots were probed with anti-pTyr 4G10. (d) Jak2 inhibition by WP1193 causes rapid reduction of pTyr177 Bcr–Abl and the Bcr–Abl protein in BaF3 cells expressing IM-resistant form of Bcr–Abl containing the T315I mutation. (e) Jak2 inhibition by WP1193 caused rapid reduction of pTyr177 Bcr–Abl in cells from a blast crisis CML patient. Monocytes from patients were isolated by Histopaque (Sigma Chem Co.,) separation and grown for 24 h in culture medium without growth factors before WP1193 treatment. (f) The level of the Y177F Bcr–Abl mutant is decreased in amount similar to wild-type Bcr–Abl as a result of Jak2 inhibition. 32D cells were transfected with either Y177F BCR–ABL mutant or wild-type BCR–ABL. Cells were allowed to grow in the absence of IL-3 for about 3 weeks. Cells were treated with 10 μM WP1193 for 30 min. Lysates were analyzed by western blotting with anti-Abl 8E9, anti-Tyr 177 Bcr, anti-Jak2 pTyr 1007/8 and anti-Jak2 antibodies. (g) Levels of Grb2 were rapidly reduced in anti-Abl immune complexes containing Bcr–Abl. (h) Levels of Ras GTP were rapidly reduced in 32Dp210 cells treated with Jak2 inhibitor WP1193. Experiments were performed as in Figure 4b. (i) Quantitation of RasGTP levels in 32Dp210 cells treated with Jak2 inhibitor WP1193. Quantitation was done as shown in Figure 4c.
Like TG, WP1193 was able to reduce binding of Grb2 to Bcr–Abl complexes while reducing levels of pTyr177 Bcr–Abl (Figure 5g). WP1193 rapidly reduced RAS GTP levels (Figures 5h and i) and pTyr Gab2, and STAT5 levels (Supplementary Figure 2c, e, respectively). WP1193 was a potent inhibitor of the Jak2 kinase in a test tube kinase assay (Supplementary Figure 2b) but did not inhibit the Bcr–Abl kinase (Supplementary Figure 2f) whereas IM, as expected, inhibited the Bcr–Abl kinase (Supplementary Figure 2g).
Tyr177 Y to F mutant behaves as wild-type Bcr–Abl with respect to Jak2 inhibition
We compared the disappearance of Y177F Bcr–Abl mutant with wild-type Bcr–Abl in 32D cells transduced with either wild-type or mutant BCR–ABL. The results indicate that Jak2 inhibition by WP1193 for 30 min caused similar levels of Bcr–Abl disappearance in both mutant and wild-type forms (Figure 5f). Moreover, as expected, Tyr177 phosphorylation was not detected in the Y177F mutant (Figure 5f). These results support the concept that Tyr177 is just one of possibly several Jak2 phosphorylation sites (Tyr360 being another, see Supplementary Table 1), and that phosphorylation of these sites is necessary to maintain Bcr–Abl in a functional state.
Jak2 inhibition reduced tumorgenicity in mouse models
As WP1193 was a more potent Jak2 inhibitor than TG, we tested the effects of WP1193 on the growth of tumors induced by IM-resistant K562-R cells. K562-R cells16 contain activated Lyn kinase, which maintains the leukemic state of the K562-R cells despite the presence of IM. Therefore, we tested the inhibitory effects of WP1193 on the growth of solid tumors induced by K562-R in a nude mouse model. Solid tumors were allowed to form for 12 days following injection of K562-R cells, and treatment with WP1193 was initiated at 12 days through day 22 (Figure 6a). The volume of solid tumors was determined following injection of WP1193 at 30 mg/kg of mouse body weight every 48 h. Solid tumor growth was significantly reduced (P-value of 0.007) over this time period (Figures 6a and b). Injection of these mice with WP1193 by intraperitoneal (i.p.) had little effect on the weights of spleen and liver tissue (Supplementary Figure 3c, d).
Jak2 inhibition by WP1193 inhibited solid tumor formation caused by IM-resistant K562 cells and strongly reduced tumors effects caused by Bcr–Abl T315I. (a) WP1193 inhibits solid tumor formation in nude mice injected subcutaneously (s.c) with 5 million K562-R cells. Five mice were injected with WP1193 (30 mg/kg) every 48 h beginning 12 days after injection of cells through 22 days. (b) Quantitation of tumor weight from five mice. (c and d) WP1193 reduced the weight of liver and spleens (right spleen in figure) in leukemic injected with Bcr–Abl T315I+ BaF3 cells (∼2.5 million cells). Mice were injected every 48 h by the i.p. route with WP1193 beginning 1 day after cell inoculation and killed 1 day after the seventh injection. (e) Reduction of spleen weight by WP1193 in mice injected with Bcr–Abl T315I BaF3 cells. (f) Model describing how Jak2 regulates signaling in CML cells.
We next compared the effects of WP1193 at 30 mg/kg (i.p.) on the oncogenic effects of IM-resistant T315I Bcr–Abl+ 32D cells injected intravenous (i.v.) in the nude mouse model. The mice developed large intestinal tumors over a 2-week period (Supplementary Figure 3a), which were largely prevented by WP1193 treatment (Supplementary Figure 3b, Figures 6c–e). Spleen and liver weights were reduced (Figures 6c–e), and spleens were enlarged in the diseased mice compared with WP1193-treated mice (Figures 6d and e).
Importantly, spleens and livers of mice not injected with leukemia cells were not significantly affected in weight by injection of WP1193 over a 3-week period (Figures 6c and e); all of these drug-treated mice survived and showed little evidence of acute toxicity as the mice appeared healthy.
Jak2 inhibition induced apoptosis in CD34+ cells from blast crisis IM-resistant CML patients and cells from chronic phase IM-resistant CML patients
We harvested CD34+ progenitor cells from a patient with blast crisis CML. Fractionated cells were treated with either IM or TG. After 48 h, cells were analyzed by annexin/PI flow cytometry. TG at 5 μM concentration induced 80% apoptosis whereas IM at 5–10 μM concentration had little apoptotic-inducing activity (Supplementary Figure 4a); (the same patient cells used in Figure 3f). All of our previous studies had shown that Jak2 inhibition20, 21 induced apoptosis in cells from blast crisis CML patients. We also examined cells from chronic phase CML patients who were resistant to IM. Treatment of the chronic phase cells with 2.5−10 μM TG induced high levels of apoptosis whereas IM treatment had little effect in these samples (Supplementary Figure 4b–d). Cells from one chronic phase patient were partially sensitive to IM but these cells were almost completely killed by 5 μM TG (Supplementary Figure 4b). Cells from an accelerated phase CML patient, although resistant to IM, were quite sensitive to Jak2 inhibition with either TG or WP1193 (Supplementary Figure 4e). We note that BaF3 cells expressing the T315I form of Bcr–Abl, although resistant to IM as expected, were quite sensitive to TG at 5 μM and above (Supplementary Figure 4f).
Discussion
Our previous findings indicate that Jak2 is a critical signaling molecule in CML.20, 21 The most pertinent of these findings is that AG490, an inhibitor of Jak2, induced apoptosis in IM-resistant Bcr–Abl+ cell lines including BaF3 cells expressing the gate keeper IM-resistant mutant T315I13 of Bcr–Abl.20 To pursue the effects of Jak2 inhibition further, we performed experiments with Jak2-specific short hairpin RNAs in three different CML cell lines and in 32Dp210 cells expressing Jak2-specific small interfering RNAs. We made a surprising finding that Jak2 knockdown caused a disappearance of Bcr–Abl from the lysate. The mechanism of this Jak2 inhibition effect on Bcr–Abl is unknown, but is under study.
Another effect of Jak2 inhibition in Bcr–Abl+ cells is the reduction of phosphorylation of Tyr177 of Bcr–Abl. Recombinant Jak2 (JH1) readily phosphorylated Tyr177 in a Bcr peptide, and this phosphorylation was strongly inhibited by a selective Jak2 inhibitor TG101209 but not by IM. Tyr177 phosphorylation in this system was also inhibited by a new Jak2 inhibitor, WP1193. Although Jak2 inhibition leads to reduction of pTyr 177 Bcr–Abl in Bcr–Abl+ cell lines and in cells from blast crisis patients, this whole-cell effect is less clear as Jak2 inhibition also decreased levels of the Bcr–Abl protein. However, in vitro immune complex kinase assays showed that Jak2 inhibition did not reduce levels of Bcr–Abl in immune complexes but strongly inhibited phosphorylation of Tyr177. Thus, our hypothesis is that Jak2 inhibition decreases phosphorylation of Tyr177 within Bcr–Abl and possibly other Tyr residues within Bcr–Abl. In this regard, there are eight consensus Jak2 phosphorylation sites (YxxV/L/I) within the Bcr portion of Bcr–Abl (b3a2) of which Tyr177 is one such site (Supplementary Table 1). We propose that decreases in Tyr phosphorylation renders Bcr–Abl insoluble in the non-ionic detergent extraction buffer normally used to solubilize Bcr–Abl. This insolubility may be caused by the destruction of the network structure that maintains leukemic signaling in Bcr–Abl+ cells.22 We have shown that another Jak2 inhibitor, which also inhibits the Bcr–Abl kinase, also causes rapid disappearance of Bcr–Abl. Importantly, this dual kinase inhibitor (ON 044580) causes the disruption of the HSP90 structure that houses Bcr–Abl and Jak2 within and other signaling components of the Bcr–Abl/Jak2 pathway.22
Of interest, CD34+ cells from CML blast crisis patients and from CD34+ cord blood cells transduced with BCR–ABL also showed reduction of Bcr–Abl and pTyr177 Bcr–Abl levels upon Jak2 inhibition, indicating that the dominant effects of Jak2 inhibition also occur in early progenitor cells.
Downregulation of Ras activation and the initial stage of PI-3 kinase activation (reduction of pTyr Gab2), and the inhibition of Tyr phosphorylation of STAT5 following Jak2 inhibition suggests that the Jak2 tyrosine kinase is the critical tyrosine kinase that drives major signaling pathways in the leukemic cell. These results partially explain why Jak2 inhibition can overcome IM resistance (Supplementary Figure 4, 5).20, 21 What remains is whether the tyrosine kinase function of Bcr–Abl is needed to activate the Jak2 kinase. Our studies indicate that Bcr–Abl alone does not lead to Jak2 activation, as addition of the IL-3 receptor is required.18
It is of interest that the Jak2 kinase seems to be involved in phosphorylation of Tyr 360 of Bcr–Abl. Our studies with Bcr serine kinase indicate that Tyr 360 is a critical regulator of Bcr serine/threonine kinase activity,32, 33 and that phosphorylation of Tyr360 of Bcr downregulates its serine/threonine kinase activity.32, 33 Bcr resembles kinases such as pyruvate kinase, which is known to require a free tyrosine residue to maintain its kinase activity.34 The fact that Y177F mutant of Bcr–Abl is similar to wild-type Bcr–Abl in response to Jak2 inhibition, suggests that Tyr177 is one of several possible Jak2 phosphorylation sites (including Tyr360) in Bcr–Abl necessary to maintain functional Bcr–Abl levels (Figure 5f).
Transmission of downstream signals induced by IL-3 may be enhanced by Jak1 interaction with Jak2.30 In this regard, we found that a putative pan Jak kinase inhibitor (WP1193) appeared to be more potent in reducing levels of Bcr–Abl and reducing levels of pTyr177 in Bcr–Abl+ cells compared with the selective Jak2 kinase inhibitor (TG101209).35 We note that WP1193, despite its considerably less in vitro potency for phosphorylating Tyr177 in kinase assays compared with TG (approximately 100-fold), still maintained similar or greater potency to reduce Jak2 effects in intact cells expressing Bcr–Abl. It is possible that WP1193's greater in vivo potency may be because of its ability to inhibit Jak1 as well Jak2 inside Bcr–Abl+ cells, although other explanations are possible.
Because of the apparent increased overall potency of WP1193 compared with TG101209, we chose to test WP1193 for its effects on inhibition of Bcr–Abl's oncogenic effects in mouse tumor models. The results indicate that WP1193 was a potent inhibitor of solid tumor formation induced by CML line K562-R and a strong inhibitor of oncogenic effects of IM-resistant T315I in nude mice. Moreover, WP1193 had no observable toxic effects on mice injected with WP1193 only over a 2-week period.
Importantly, Jak2 inhibition was effective in apoptosis induction in IM-resistant blast crisis cells, IM-resistant accelerated phase cells, IM-resistant chronic phase and CD34+ progenitors from accelerated and blast crisis patients.
We conclude from these experiments that Jak2 is the main tyrosine kinase that controls signaling in Bcr–Abl+ cells, as Jak2 appears to use the Bcr–Abl protein as a platform to activate the Ras and PI-3 kinase pathways by phosphorylating Tyr 177 within the Bcr portion of Bcr–Abl. Jak2 inhibition also strongly reduced STAT5 tyrosine phosphorylation, possibly because Jak2 inhibition may reduce functional Bcr–Abl levels. A model summarizes our current and past findings on Jak2 functions in Bcr–Abl+ leukemia (Figure 6f). Importantly, leukemia cells expressing either IM-resistant forms of Bcr–Abl or having other forms of drug resistance (such as in blast crisis) undergo apoptosis on exposure to Jak2 inhibitors20, 21 (Supplementary Figure 4), suggesting that Jak2 inhibitors have potential for treatment of drug-resistant CML.
References
Wilson-Rawls J, Xie SH, Liu J, Laneuville P, Arlinghaus RB . P210 Bcr-Abl interacts with the interleukin-3 βc subunit and constitutively induces its tyrosine phosphorylation. Cancer Res 1996; 56: 3426–3430.
Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE et al. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene 2001; 20: 6188–6195.
Xie S, Lin H, Sun T, Arlinghaus RB . Jak2 is involved in c-Myc induction by Bcr-Abl. Oncogene 2002; 21: 7137–7146.
Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 1998; 92: 3780–3792.
Puil L, Liu J, Gish G, Mbamalu G, Bowtell D, Pelicci PG et al. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J 1994; 13: 764–773.
Sattler M, Mohi MG, Pride YB, Quinnan LR, Malouf NA, Podar K et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 2002; 1: 479–492.
Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA . JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 2004; 18: 189–218.
Coppo P, Flamant S, De Mas V, Jarrier P, Guillier M, Bonnet ML et al. BCR-ABL activates STAT3 via JAK and MEK pathways in human cells. Br J Haematol 2006; 134: 171–179.
Ilaria Jr RL, Hawley RG, Van Etten RA . Dominant negative mutants implicate STAT 5 in myeloid cell proliferation and neutrophil differentiation. Blood 1999; 93: 4154–4166.
Klejman A, Schreiner SJ, Nieborowska-Skorska M, Slupianek A, Wilson M, Smithgall TE et al. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. EMBO J 2002; 21: 5766–5774.
Zhang X, Subrahmanyam R, Wong R, Gross AW, Ren R . The NH (2)-terminal coiled-coil domain and tyrosine 177 play important roles in induction of a myeloproliferative disease in mice by Bcr-Abl. Mol Cell Biol 2001; 21: 840–853.
Chu S, Li L, Singh H, Bhatia R . BCR-tyrosine 177 plays an essential role in Ras and Akt activation and in human hematopoietic progenitor transformation in chronic myelogenous leukemia. Cancer Res 2007; 67: 7045–7053.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–880.
Branford S, Rudzki Z, Parkinson I, Grigg A, Taylor K, Seymour JF et al. Real-time quantitative PCR analysis can be used as a primary screen to identify patients with CML treated with imatinib who have BCR-ABL kinase domain mutations. Blood 2004; 104: 2926–2932.
Saffroy R, Lemoine A, Brezillon P, Frenoy N, Delmas B, Goldschmidt E et al. Real-time quantitation of bcr-abl transcripts in haematological malignancies. Eur J Haematol 2000; 65: 258–266.
Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 2003; 101: 690–698.
Donato NJ, Wu JY, Stapley J, Lin H, Arlinghaus R, Aggarwal BB et al. Imatinib mesylate resistance through BCR-ABL independence in chronic myelogenous leukemia. Cancer Res 2004; 64: 672–677.
Tao WJ, Lin H, Sun T, Samanta AK, Arlinghaus R . BCR-ABL oncogenic transformation of NIH 3T3 fibroblasts requires the IL-3 receptor. Oncogene 2008; 27: 3194–3200.
Tao W, Samanta AK, Priebe W, Arlinghaus RB . Enhanced Jak2 activation correlates with IL-3 receptor β Chain expression leading to phosphorylation of tyrosine 177 of Bcr-Abl. Blood (51th Am Soc Hematol Annu Meet Abst) 2009; 114: 854 (abstract 2170).
Samanta AK, Lin H, Sun T, Kantarjian H, Arlinghaus RB . Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res 2006; 66: 6468–6472.
Samanta AK, Chakraborty SN, Wang Y, Kantarjian H, Sun X, Hood J et al. Jak2 inhibition deactivates Lyn kinase through the SET-PP2A-SHP1 pathway, causing apoptosis in drug-resistant cells from chronic myelogenous leukemia patients. Oncogene 2009; 28: 1669–1681.
Samanta AK, Chakraborty SN, Wang Y, Schlette E, Reddy EP, Arlinghaus RB . Destabilization of Bcr-Abl/Jak2 Network by a Jak2/Abl kinase inhibitor ON044580 overcomes drug resistance in blast crisis chronic myelogenous leukemia (CML). Genes Cancer 2010; 1: 346–359.
Stuart SA, Minami Y, Wang JY . The CML stem cell: evolution of the progenitor. Cell Cycle 2009; 8: 1338–1343.
Minami Y, Stuart SA, Ikawa T, Jiang Y, Banno A, Hunton IC et al. BCR-ABL-transformed GMP as myeloid leukemic stem cells. Proc Natl Acad Sci USA 2008; 105: 17967–17972.
Nicholson E, Holyoake T . The chronic myeloid leukemia stem cell. Clin Lymphoma Myeloma 2009; 9 (Suppl 4): S376–S381.
Jamieson CH . Chronic myeloid leukemia stem cells. Hematology Am Soc Hematol Educ Program 2008, 436–442.
Jorgensen HG, Holyoake TL . Characterization of cancer stem cells in chronic myeloid leukaemia. Biochem Soc Trans 2007; 35 (Pt 5): 1347–1351.
Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 2008; 134: 496–507.
Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN . Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol 1997; 17: 2497–2501.
Huang HM, Lin YL, Chen CH, Chang TW . Simultaneous activation of JAK1 and JAK2 confers IL-3 independent growth on Ba/F3 pro-B cells. J Cell Biochem 2005; 96: 361–375.
Argetsinger LS, Kouadio JL, Steen H, Stensballe A, Jensen ON, Carter-Su C . Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol Cell Biol 2004; 24: 4955–4967.
Liu J, Wu Y, Ma GZ, Lu D, Haataja L, Heisterkamp N et al. Inhibition of Bcr serine kinase by tyrosine phosphorylation. Mol Cell Biol 1996; 16: 998–1005.
Wu Y, Liu J, Arlinghaus RB . Requirement of two specific tyrosine residues for the catalytic activity of Bcr serine/threonine kinase. Oncogene 1998; 16: 141–146.
Maru Y, Witte ON . The BCR gene encodes a novel serine/threonine kinase activity within a single exon. Cell 1991; 67: 459–468.
Pardanani A, Hood J, Lasho T, Levine RL, Martin MB, Noronha G et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia 2007; 21: 1658–1668.
Acknowledgements
This work was supported in part by a research grant from the National Cancer Institute, USA CA49639. TG101209 was provided as a gift from TargeGen Inc., San Diego, CA, USA (now part of Sanofi-Aventis, Bridgewater, NJ, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
WP is a senior inventor on patent covering WP1193 and has financial interest in (Moleculin, LLC) that licensed his patent from MD Anderson Cancer Center. He is also a member of Moleculin's SAB and has an SRA covering discovery on novel inhibitors.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Samanta, A., Perazzona, B., Chakraborty, S. et al. Janus kinase 2 regulates Bcr–Abl signaling in chronic myeloid leukemia. Leukemia 25, 463–472 (2011). https://doi.org/10.1038/leu.2010.287
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2010.287
Keywords
This article is cited by
-
Chronic myeloid leukemia stem cells: targeting therapeutic implications
Stem Cell Research & Therapy (2021)
-
Expression differences of miR-142-5p between treatment-naïve chronic myeloid leukemia patients responding and non-responding to imatinib therapy suggest a link to oncogenic ABL2, SRI, cKIT and MCL1 signaling pathways critical for development of therapy resistance
Experimental Hematology & Oncology (2020)
-
DYRK2 controls a key regulatory network in chronic myeloid leukemia stem cells
Experimental & Molecular Medicine (2020)
-
Die CML und das Problem der Stammzellpersistenz
InFo Hämatologie + Onkologie (2020)
-
Chronic myeloid leukemia stem cells
Leukemia (2019)