Abstract
Purpose
To investigate factors associated with good response to intravitreal bevacizumab (IVB) in central serous chorioretinopathy (CSC) patients.
Methods
We retrospectively reviewed 42 eyes of CSC patients of symptom duration more than 3 months who received a single or multiple successive IVBs on an as-needed basis (0.05 ml, 1.25 mg). High responders (HRs) were defined as complete resolution of subretinal fluid (SRF) on spectral domain optical coherence tomography (SD-OCT). Moderate responders (MRs) were defined as SRF resolution of 50–99% of pretreatment volume and poor responders (PRs) as SRF resolution <50%. Clinical, SD-OCT, fluorescein, and indocyanine green angiography findings were analyzed to find factors associated with HR. Descriptive statistics for all demographic and clinical variables were calculated, and comparisons were made using Wilcoxon’s matched-pairs signed-rank test, the Mann–Whitney U-test for means with continuous data, Pearson’s χ2 test, and Fisher’s exact test for categorical data.
Results
The mean number of IVB was 1.9. At postoperative 1 month, there were 10 (24%) HRs, 18 (43%) MRs, and 14 (33%) PRs. At the last follow-up (the mean 8.6 months), there were 25 HRs (60%), 9 MRs (21%), and 8 PRs (19%). Thicker subfoveal choroid (P=0.036), smaller lesion diameter (P=0.019), and better baseline best-corrected visual acuity (P=0.002) predicted HRs at postoperative 1 month. HR at the last follow-up was associated with classic pattern fluorescein angiography finding.
Conclusions
Suboptimal effects of IVB on persistent CSC suggest primary IVB on selective cases with better vision, smaller lesion, and thicker choroid at baseline.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC) is one of the most common macular disorders, characterized by neurosensory retinal detachment and varying degrees of retinal pigment epithelium (RPE) change.1 The pathogenesis remains poorly understood, but it is generally accepted that stasis, ischemia, and/or inflammation of the inner choroid leads to hyperpermeability of choroidal vasculature, secondary RPE change, and neurosensory retinal detachment.2, 3, 4, 5 A variety of factors have been associated with CSC, including preceding stressful event, type A personality, elevated levels of corticosteroid, and genetic susceptibility.6, 7, 8 Spontaneous resolution of neurosensory retinal detachment was found in ~90% of patients, but some patients exhibit persistent or recurrent CSC, and never achieve full visual recovery. Various treatments, including focal photocoagulation, photodynamic therapy (PDT), anti-vascular endothelial growth factor (VEGF) intravitreal injections, corticosteroid antagonists, and acetazolamide have been used to promote the remission of persistent or recurrent CSC.9, 10, 11, 12, 13, 14
Intravitreal bevacizumab (IVB), a recombinant humanized monoclonal anti-VEGF antibody, has been reported to be effective in CSC patients with symptom durations over 3 months.9, 15, 16, 17, 18, 19, 20 In acute CSC of <3 months symptom duration, IVB may not result in therapeutic benefits compared with simply waiting for spontaneous resolution.21, 22 It is not clear why and how IVB induces the absorption of subretinal fluid (SRF) in CSC. Anti-VEGF activities, including antiangiogenic and antipermeable properties of bevacizumab, have been proposed as its general mechanism of action. But aqueous VEGF is not significantly elevated in eyes with CSC.16, 23, 24, 25 Moreover, there appears to be significant individual variation in treatment responses to IVB.9, 20 CSC encompasses a wide range of patients with varying degrees and patterns of angiographical and tomographical manifestations. Previous studies have validated the general efficacy and safety of IVB for CSC, but it is largely unknown what factors would predict favorable or unfavorable response to this treatment.
In the current study, we therefore investigated clinical outcomes of a single or multiple successive IVBs for treatment-naive CSC patients, with no previous treatment and disease duration more than 3 months, focusing on the clinical, angiographical, and tomographical prognostic factors associated with complete resolution of SRF.
Materials and methods
Design and study population
We retrospectively reviewed the medical charts of 42 eyes of 42 patients with persistent CSC of more than 3 months duration, treated with IVB (0.05 ml, 1.25 mg) at Severance Hospital, Yonsei University College of Medicine, from November 2009 to February 2014. Patients underwent comprehensive ophthalmologic examinations, including measurements of best-corrected visual acuity (BCVA), indirect ophthalmoscope, fundus photography, fluorescein angiography (FA), and spectral domain optical coherence tomography (SD-OCT) with enhanced depth imaging optical coherence tomography (EDI-OCT). The BCVA was recorded using the Snellen chart and was converted to the logarithm of the minimum angle of resolution (LogMAR) equivalents. An ancillary examination included use of indocyanine green angiography (ICGA). Criteria for exclusion were as follows: (1) patients who received any ocular or systemic treatment for CSC before IVB, such as laser photocoagulation, PDT, or systemic carbonic anhydrase inhibitor, (2) patients who had coexisting macular conditions that could induce SRF collection (eg, severe epiretinal membrane or definite vitreomacular traction, extensive macular ischemic changes, or age-related macular degeneration), (3) patients on long-term local or systemic corticosteroid treatment, which could have affected the occurrence, treatment response, and recurrence of CSC, and (4) patients with spherical equivalence >+6.0 or <–6.0 D. All the research and measurements adhered to the tenets of the Declaration of Helsinki, and the study was approved by the local Institutional Review Board/Ethics Committee.
FA and ICGA
FA and ICGA, with a confocal scanning laser ophthalmoscope, HRA2 (Heidelberg Engineering, Heidelberg, Germany), were analyzed by the primary investigator and an experienced retina specialist (GAK, CSL). The high-speed mode (resolution of 768 × 768 pixels), and a frame size of 30 × 30° with 3 mm scan depth to attain 10 μm/pixel resolution, were selected. Before dye injection, red-free images, preinjection images, and autofluorescent images were taken. The FA findings with clinical ophthalmic examination were classified as either classic CSC or diffuse retinal pigment epitheliopathy (DRPE).26 Classic CSC was defined as focal or multifocal detachments of the neurosensory retina, or RPE with single or multifocal areas of leakage at late phase on FA. DRPE was defined as geographic areas of atrophy and mottling of RPE with slow, subtle leakage on FA. Distance (μm) between the fovea center and the nearest leakage was measured manually in FA images.
ICGA images were analyzed for the presence of choroidal filling delay, choroidal hyperpermeabilty, and punctate hyperfluorescence. Choroidal filling delay was evaluated in the early-phase ICGA images, whereas choroidal hyperpermeability (focal areas of hyperfluorescence with blurred margins) and punctate hyperfluorescent spots were evaluated in the mid/late-phase ICGA images, as described previously.2, 27
Optical coherence tomography
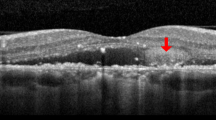
SD-OCT (Spectralis; Heidelberg Engineering), with automatic real-time tracking that could achieve data with acquisition rates of up to 40 000 axial scans/s, was used in this study. Multiple raster scans (covering 20 × 15°, multiple horizontal scans of 119 μm spacing, and at least 37 B scans) were performed. In those areas of interest in FA, raster scans of 30 μm spacing were additionally taken. Real-time averaging of multiple frames (at least 15 frames in raster scans) and noise reduction software were used in raster scans. Horizontal or vertical cross-sectional scans crossing the foveal center were also performed (maximal noise reduction with averaging of 100 frames). After confinement of lesion boundaries as basal areas of the detached retina, we measured the greatest linear diameter (GLD) manually, using calipers provided by the software of the Heidelberg system. Likewise, the height of the SRF was defined as the greatest distance from RPE to the border of the detached neurosensory retina, which was also measured manually within an area from 3 mm nasal and 3 mm temporal from the center of the fovea. EDI-OCT was used to measure subfoveal choroidal thickness from the outer portion of the hyperreflective line corresponding to the RPE, to the inner surface of the sclera at the center of the fovea.28 Measurements of the GLD, SRF height, and subfoveal choroidal thickness are described in Figure 1. The central macular thickness in nine standard, early treatment diabetic retinopathy study (ETDRS) macular-grid subfields was calculated using the bundled Heidelberg software. The ETDRS grid was manually placed, to be centered on the fovea. Volumetric analysis of SRF was also performed using a built-in segmentation-modifying tool in the same manner as a previous study.29
Measurements of the greatest linear diameter, subfoveal choroidal thickness, and height of SRF. On SD-OCT, the greatest linear diameter of lesion boundaries, as basal area of detached retina, was measured (left). Also, the height of SRF was defined as the greatest distance from the retinal pigment epithelium (RPE) to the border of the detached neurosensory retina, within an area from 3 mm nasal and 3 mm temporal from the center of the fovea (right). Subfoveal choroidal thickness was measured from the RPE to the inner surface of the sclera within an area at the center of the fovea, on enhanced depth imaging optical coherence tomography (EDI-OCT) (right).
Outcome variables
Effects of IVB were evaluated by primary outcome variables including BCVA, GLD, SRF height, SRF volume, central macular thickness, and secondary outcome variables including subfoveal choroidal thickness. High responders (HRs) were defined as patients who achieved complete resolution of SRF on SD-OCT following IVB. Moderate responders (MRs) included patients whose SRF volume decreased more than 50% from the pretreatment volume, but not completely on SD-OCT following IVB. Poor responders (PRs) were defined as patients whose SRF volume decreased <50% of pretreatment volume on SD-OCT following IVB. Statistical analysis was used to correlate demographic, angiographic, and topographic factors that were associated with complete resolution of SRF after IVB. These factors included demographic characteristics (age, sex, disease duration before injection, spherical equivalent, and visual acuity), FA features (CSC type and distance from fovea to the nearest leakage site), ICGA features (presence of choroidal hyperpermeability, choroidal filling delay, and punctate hyperfluorescent spots), and SD-OCT findings (GLD, SRF height, SRF volume, central macular thickness, and subfoveal choroidal thickness).
Statistical analysis
Descriptive statistics for all demographic and clinical variables were calculated, and comparisons were made using Wilcoxon’s matched-pairs signed-rank test, the Mann–Whitney U-test for means with continuous data, Pearson’s χ2 test, and Fisher’s exact test for categorical data. Statistical analyses were performed using SPSS 18.0 for windows (SPSS/IBM Corporation, Chicago, IL, USA). Differences with P<0.05 were considered statistically significant.
Results
Forty-two eyes of 42 subjects were included. The mean age was 49.4±11.1 years. Twenty-six (62%) patients were men. The mean duration of symptoms was 6.8±10.5 months. During the mean follow-up of 8.6±7.7 months, the mean number of IVBs was 1.9±0.8. None of the eyes developed serious injection-related complications such as endophthalmitis. At last follow-up, there were 25 (60%) patients with complete resolution (HR) of SRF, 9 (21%) patients with moderate response (MR), and 8 (19%) patients with poor response (PR). The mean initial LogMAR BCVA was 0.35±0.34 (Snellen equivalent of 0.45), which did not show significant improvement at the last follow-up (LogMAR 0.32±0.36 or Snellen equivalent of 0.48; P=0.359). Despite the lack of visual improvement, GLD of SRF significantly decreased by 73.1% (P<0.001), SRF height by 79.6% (P<0.001), SRF volume by 81.3% (P<0.001), central macular thickness by 37.3% (P<0.001), and subfoveal choroidal thickness by 4.6% (P=0.008) (Table 1). Subgroup analysis showed that visual improvement was seen in the HR group only (LogMAR BCVA of 0.30±0.30 to 0.20±0.28; P=0.015). The recurrence after complete SRF resolution occurred in 8 (44.4%) patients at various intervals between 1.25 and 24 months.
We further analyzed the clinical outcome at 1 month after the first IVB to better understand the short-term effect of bevacizumab on CSC patients. After a single session of IVB, 10 (24%) patients achieved HR, whereas 18 (43%) patients showed MR, and 14 (33%) patients showed PR. Overall, there was no visual improvement (LogMAR BCVA of 0.35±0.34 to 0.38±0.37; P=0.699). However, GLD of SRF significantly decreased by 38.0% (P<0.001), SRF height by 58.7% (P<0.001), SRF volume by 51.1% (P<0.001), and central macular thickness by 30.5% (P<0.001). Subfoveal choroidal thickness did not change (Table 1). Of the 18 MRs, 11 patients received a second IVB, which resulted in 7 (64%) HR, 1 (9%) MR, and 3 (27%) PR after 1 month. Of the 14 PRs after the first IVB, 8 patients received the second IVB, which resulted in 1 (12.5%) HR, 2 (25%) MR, and 5 (62.5%) PR.
Analysis of demographic, angiographic, and topographic features was conducted between HR and MR/PR patients (Table 2). Better initial BCVA (P=0.002), smaller GLD of SRF (P=0.019), and thicker subfoveal choroidal thickness (P=0.036) predicted complete resolution of SRF at 1 month after a single session of IVB. The only FA type of classic CSC was associated with complete resolution of SRF at final follow-up with marginal statistical significance (P=0.041). Examples of association of thicker subfoveal choroidal thickness with favorable response to IVB at postoperative 1 month as opposed to thin subfoveal choroidal thickness are displayed in Figures 2 and 3.
A 35-year-old man with central serous chorioretinopathy (CSC) who showed high response after single intravitreal bevacizumab treatment. Fluorescein angiography (a), indocyanine green angiography (b), and SD-OCT at baseline with thick choroid (614 μm, white arrow) (c). Complete resolution of SRF was noted at 1 month after intravitreal bevacizumab (d).
A 30-year-old man with CSC demonstrating poor response after single intravitreal bevacizumab treatment. Fluorescein angiography (a), indocyanine green angiography (b), SD-OCT at baseline with relatively thin choroid (289 μm, white arrow) (c). Slightly decreased, but remaining SRF was noted at 1 month after intravitreal bevacizumab (d).
Discussion
CSC usually resolves spontaneously within 3 months,30 but because the neurosensory detachment can cause significant photoreceptor damage and/or RPE atrophy, treatments such as focal laser photocoagulation or PDT have long been applied to CSC with persistent SRF. PDT has shown favorable outcomes in chronic CSC patients, but it can result in significant complications such as choroidal ischemia and RPE atrophy.31, 32 Focal laser photocoagulation is an effective treatment option, but it is difficult to apply laser treatment if the leakage point is close to the fovea or subtle as in DRPE-type CSC. In addition, iatrogenic choroidal neovascularization is a worrisome complication of focal laser treatment.1, 33 IVB can be an attractive option as an initial treatment for chronic CSC, and in recent studies has shown varying degrees of efficacy without serious complications.9, 15, 17, 18, 19, 20
In the present study, 24% of CSC patients with persistent SRF showed complete resolution of SRF within 1 month after a single session of IVB. At the mean follow-up of 8.6 months, 60% of patients achieved complete resolution with mean IVBs of 1.9. Lim and Kim9 have reported a higher rate of complete SRF resolution after IVB of CSC patients, with symptom duration more than 3 months as 57.5% within 1 month, and 82.5% within 3 months following the first IVB. The reason for this difference is not clear, but our study may have included patients with more chronic disease, as the baseline BCVA was slightly lower (LogMAR 0.35 vs 0.25) and the mean symptom duration was slightly longer in our study (6.8 vs 3.6 months for the resolution group, and 4.2 months for the persistent group).
We found that thicker subfoveal choroid, smaller GLD of SRF, and better initial BCVA predicted complete resolution of SRF following single IVB treatment. Considering the high self-remission rate of CSC and short half-life of bevacizumab that remains in the eye for 4 to 6 weeks,34, 35 we chose to analyze clinical results at 1 month after single IVB treatment. Association between thicker choroid and better treatment response to bevacizumab in CSC was an interesting finding and, to the best of knowledge, has not been reported. This finding is in agreement with the study by Lim and Kim9 that reported a higher rate of CSC resolution in groups with more intense hyperfluorescence or choroidal vascular hyperpermeability on ICGA after IVB. Choroidal thickness is related to choroidal vascular hyperpermeability in CSC,36 so a thicker choroid may indicate prominent choroidal vascular hyperpermeability. It has been hypothesized that IVB results in SRF resolution in CSC by decreasing choroidal hyperpermeability, which subsequently decreases leakage at the RPE.19, 23, 37 Electron microscopic studies in rat and rabbits demonstrated that IVB can reduce fenestrations of choriocapillaris,38, 39 but anti-VEGF drugs (bevacizumab, ranibizumab) had minimal effects on permeability or selectivity of RPE tight junctions.40 Choroidal hyperpermeability was present in all cases with available ICGA images in the present study, but we did not show the intensity of hyperfluorescence, owing to difficulty in grouping with a certain level of objectivity. In addition, choroidal thickness measured on EDI-OCT is a noninvasive and more objective measurement, which may have more clinical values. Other ICGA features of CSC, including the presence of punctate hyperfluorescence and choroidal filling delay, were not associated with treatment response to IVB in the present study.
There have yet been no established treatment schemes of IVB for idiopathic persistent CSC. Because single IVB treatment resulted in only a 24% rate of complete resolution, it is advisable to consider IVB selectively as a primary treatment for persistent CSC with good BCVA, small GLD, and thicker choroid. If the response was moderate, successive IVB treatments could be considered, as we found a 64% rate of complete resolution in these cases. However, if the response is poor after the first IVB, successive IVB treatments would not be a good choice, and other options such as PDT or focal laser photocoagulation should be considered. The relatively high recurrence rate of 44.4% suggests that IVB may not have as long-lasting effect as PDT, and that patients should therefore be monitored for recurrence on a regular basis.41
Retrospective design, lack of control groups, and the lack of controlled treatment criteria are important limitations of the current study. Several study parameters, including the distance from fovea to the nearest leakage site, GLD and height of SRF, and subfoveal choroidal thickness were measured manually, owing to the absence of automated software. Despite these limitations, we demonstrated that favorable short-term response to IVB in CSC could be predicted in eyes with thicker subfoveal choroid, smaller lesion size, and better BCVA at baseline. This finding and our clinical results may aid clinicians to better select and schedule treatment plans in managing chronic CSC patients.

References
Wang M, Munch IC, Hasler PW, Prunte C, Larsen M . Central serous chorioretinopathy. Acta Ophthalmol 2008; 86 (2): 126–145.
Prunte C . Indocyanine green angiographic findings in central serous chorioretinopathy. Int Ophthalmol 1995; 19 (2): 77–82.
Prunte C, Flammer J . Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 1996; 121 (1): 26–34.
Hayashi K, Hasegawa Y, Tokoro T . Indocyanine green angiography of central serous chorioretinopathy. Int J Ophthalmol 1986; 9 (1): 37–41.
Yannuzzi LA . Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol 2010; 149 (3): 361–363.
Bouzas EA, Karadimas P, Pournaras CJ . Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol 2002; 47 (5): 431–448.
Weenink AC, Borsje RA, Oosterhuis JA . Familial chronic central serous chorioretinopathy. Ophthalmologica 2001; 215 (3): 183–187.
Yannuzzi LA . Type A behavior and central serous chorioretinopathy. Trans Am Ophthalmol Soc 1986; 84: 799–845.
Lim JW, Kim MU . The efficacy of intravitreal bevacizumab for idiopathic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2011; 249 (7): 969–974.
Ruiz-Moreno JM, Lugo FL, Armada F, Silva R, Montero JA, Arevalo JF et al. Photodynamic therapy for chronic central serous chorioretinopathy. Acta Ophthalmol 2010; 88 (3): 371–376.
Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa D, Huang SJ et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2003; 23 (3): 288–298.
Burumcek E, Mudun A, Karacorlu S, Arslan MO . Laser photocoagulation for persistent central serous retinopathy: results of long-term follow-up. Ophthalmology 1997; 104 (4): 616–622.
Pikkel J, Beiran I, Ophir A, Miller B . Acetazolamide for central serous retinopathy. Ophthalmology 2002; 109 (9): 1723–1725.
Nielsen JS, Jampol LM . Oral mifepristone for chronic central serous chorioretinopathy. Retina 2011; 31 (9): 1928–1936.
Artunay O, Yuzbasioglu E, Rasier R, Sengul A, Bahcecioglu H . Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res 2010; 35 (2): 91–98.
Huang WC, Chen WL, Tsai YY, Chiang CC, Lin JM . Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye (Lond) 2009; 23 (2): 488–489.
Inoue M, Kadonosono K, Watanabe Y, Kobayashi S, Yamane S, Arakawa A . Results of one-year follow-up examinations after intravitreal bevacizumab administration for chronic central serous chorioretinopathy. Ophthalmologica 2011; 225 (1): 37–40.
Lee ST, Adelman RA . The treatment of recurrent central serous chorioretinopathy with intravitreal bevacizumab. J Ocul Pharmacol Ther 2011; 27 (6): 611–614.
Lim SJ, Roh MI, Kwon OW . Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina 2010; 30 (1): 100–106.
Schaal KB, Hoeh AE, Scheuerle A, Schuett F, Dithmar S . Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol 2009; 19 (4): 613–617.
Seong HK, Bae JH, Kim ES, Han JR, Nam WH, Kim HK . Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica 2009; 223 (5): 343–347.
Lim JW, Ryu SJ, Shin MC . The effect of intravitreal bevacizumab in patients with acute central serous chorioretinopathy. Korean J Ophthalmol 2010; 24 (3): 155–158.
Torres-Soriano ME, Garcia-Aguirre G, Kon-Jara V, Ustariz-Gonzales O, Abraham-Marin M, Ober MD et al. A pilot study of intravitreal bevacizumab for the treatment of central serous chorioretinopathy [case reports]. Graefes Arch Clin Exp Ophthalmol 2008; 246 (9): 1235–1239.
Shin MC, Lim JW . Concentration of cytokines in the aqueous humor of patients with central serous chorioretinopathy. Retina 2011; 31 (9): 1937–1943.
Lim JW, Kim MU, Shin MC . Aqueous humor and plasma levels of vascular endothelial growth factor and interleukin-8 in patients with central serous chorioretinopathy. Retina 2010; 30 (9): 1465–1471.
Lee CS, Kang EC, Lee KS, Byeon SH, Koh HJ, Lee SC . Central serous chorioretinopathy after renal transplantation. Retina 2011; 31 (9): 1896–1903.
Tsujikawa A, Ojima Y, Yamashiro K, Ooto S, Tamura H, Nakagawa S et al. Punctate hyperfluorescent spots associated with central serous chorioretinopathy as seen on indocyanine green angiography. Retina 2010; 30 (5): 801–809.
Margolis R, Spaide RF . A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 2009; 147 (5): 811–815.
Ahn SE, Oh J, Oh JH, Oh IK, Kim SW, Huh K . Three-dimensional configuration of subretinal fluid in central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2013; 54 (9): 5944–5952.
Gass JD . Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967; 63 (3): Suppl 1–139.
Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, Viestenz A, Naumann GO . Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol 2002; 120 (6): 835–844.
Koytak A, Erol K, Coskun E, Asik N, Ozturk H, Ozerturk Y . Fluorescein angiography-guided photodynamic therapy with half-dose verteporfin for chronic central serous chorioretinopathy. Retina 2010; 30 (10): 1698–1703.
Nomura Y, Obata R, Yanagi Y . Intravitreal bevacizumab for iatrogenic choroidal neovascularization due to laser photocoagulation in central serous chorioretinopathy. Jpn J Ophthalmol 2012; 56 (3): 245–249.
Krohne TU, Eter N, Holz FG, Meyer CH . Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol 2008; 146 (4): 508–512.
Diabetic Retinopathy Clinical Research N, Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007; 114 (10): 1860–1867.
Jirarattanasopa P, Ooto S, Tsujikawa A, Yamashiro K, Hangai M, Hirata M et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology 2012; 119 (8): 1666–1678.
Li XJ, Zhang JS . Intravitreal bevacizumab injection for chronic central serous chorioretinopathy. Chin Med J (Engl) 2010; 123 (15): 2145–2147.
Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU et al. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol 2007; 143 (6): 995–1002.
Shimomura Y, Hirata A, Ishikawa S, Okinami S . Changes in choriocapillaris fenestration of rat eyes after intravitreal bevacizumab injection. Graefes Arch Clin Exp Ophthalmol 2009; 247 (8): 1089–1094.
Peng S, Adelman RA, Rizzolo LJ . Minimal effects of VEGF and anti-VEGF drugs on the permeability or selectivity of RPE tight junctions. Invest Ophthalmol Vis Sci 2010; 51 (6): 3216–3225.
Yamashita A, Shiraga F, Shiragami C, Shirakata Y, Fujiwara A . Two-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2013; 155 (1): 96–102.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
One of the authors, Dr Hyoung Jun Koh, has financial conflict about ranibizumab. He has received grants for clinical research from Novartis, served as an advisor for Novartis, Bayer, and Allergan, and served as a speaker for Santen. The other authors have no proprietary or commercial interest in any materials discussed in this article.
Additional information
Partly presented at the 2013 Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, May 2013 Seattle, WA, USA.
Rights and permissions
About this article
Cite this article
Kim, G., Rim, T., Lee, S. et al. Clinical characteristics of responders to intravitreal bevacizumab in central serous chorioretinopathy patients. Eye 29, 732–741 (2015). https://doi.org/10.1038/eye.2015.58
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2015.58
This article is cited by
-
Exosomal miR-184 in the aqueous humor of patients with central serous chorioretinopathy: a potential diagnostic and prognostic biomarker
Journal of Nanobiotechnology (2023)
-
Leitlinien für die Betreuung und Behandlung von Patienten mit Chorioretinopathia centralis serosa
Spektrum der Augenheilkunde (2016)