Abstract
Purpose
The purpose of this study is to determine the efficacy of half-fluence photodynamic therapy (PDT) depending on the degree of hyperfluorescence based on indocyanine green angiography (ICGA) for treatment of chronic central serous chorioretinopathy (CSC).
Methods
We conducted a prospective study of 30 eyes of 30 patients with chronic CSC. Half-fluence PDT (25 J/cm2 for 83 s) with ICGA guidance was applied to the area of choroidal hyperpermeability. The baseline middle-phase ICGA findings were classified as intense or weak hyperfluorescence depending on the degree of hyperpermeability from choriocapillaris. Changes in mean best-corrected visual acuity, resolution of subretinal fluid, recurrence rate, and complications were compared between the two groups.
Results
The baseline ICGA findings showed intense hyperfluorescence in 16 eyes (53.3%) and weak hyperfluorescence in 14 eyes (46.7%). Subretinal fluid showed complete resolution in both the groups 1 month after a single application of half-fluence PDT. Recurrence of subretinal fluid was observed in one of 14 eyes (7.1%) with weak hyperfluorescence and in no eyes (0%) with intense hyperfluorescence. No statistically significant difference in the rate of recurrence was observed between the two groups.
Conclusion
Half-fluence PDT appears to be an effective and safe treatment option for patients with chronic CSC regardless of the degree of hyperfluorescence based on ICGA. According to these findings, choroidal hyperpermeability, rather than dysfunction of retinal pigment epithelium, might be more important as primary pathogenesis of chronic CSC.
Similar content being viewed by others
Introduction
Central serous chorioretinopathy (CSC) has been regarded as a condition characterized by a pathologic change in the retinal pigment epithelium (RPE) and accompanying serous detachment of neurosensory retina.1, 2, 3 In the past, because of the observance of characteristic fluorescein leakage through the RPE on fluorescein angiography (FA), defect or decompensation of the RPE was deemed responsible for detachment of the neurosensory retina.4 However, since the observance of abnormally congested and dilated choroidal veins, and capillaries on indocyanine green angiography (ICGA), it is now generally accepted that abnormal choroidal hyperpermeability is the primary pathogenic mechanism of CSC.5, 6
Unlike acute CSC, chronic CSC causes such complications as diffuse RPE atrophy,7 subretinal fibrosis and exudate,8 cystoid macular edema,9 or choroidal neovascularization (CNV),10 which leads to poorer prognosis of visual acuity. Thus, various treatment modalities for management of chronic CSC have been attempted. Laser photocoagulation, one of the existing treatments, can cause certain complications, including scotoma, secondary CNV, and an extension of RPE atrophy to the fovea; therefore, it is not widely used.11 Since recognition of the involvement of abnormal choroidal hyperpermeability in the pathogenesis of CSC, attempts have been made to use intravitreal bevacizumab injection and photodynamic therapy (PDT), which are used in treatment of CNV, for treatment of chronic CSC.12, 13, 14 In particular, treatment with bevacizumab is expected to result in improvement of symptoms of CSC through direct inhibition of vascular endothelial growth factor, a representative promoter of vascular permeability; however, an insufficient number of studies on the long-term therapeutic effects of bevacizumab have been reported, and, in many cases, due to the high rate of recurrence of CSC, multiple injections are reportedly needed.12
PDT for treatment of chronic CSC has been reported to be efficacious in improving visual acuity, reducing subretinal fluid, and decreasing recurrence rate in most patients through contracting and transiently occluding choriocapillaries thereby reducing choroidal exudation and inducing choriocapillary remodeling.15, 16 Recently, because ICGA is thought to be more useful in confirming the diagnosis and guiding laser therapy than FA, ICGA-guided PDT has been commonly performed on documented areas of choroidal hyperpermeability on ICGA.17, 18, 19, 20 Some studies have demonstrated that ICGA in eyes with CSC showed diverse intensities of choroidal hyperpermeability overlying the site of fluorescein leakage,1, 9 and evaluation of the association between ICGA findings and the efficacy of PDT in chronic CSC has been attempted.19, 20 However, the results have been conflicting. Inoue et al20 claimed that the success rate of PDT is dependent on the degree of choroidal hyperpermeability on ICGA, demonstrating more frequent recurrence in eyes with intermediate hyperfluorescence than eyes with intense hyperfluorescence. On the other hand, Nicolo et al19 suggested that eyes with intense hyperfluorescence on ICGA showed a higher rate of recurrence (13.0%) compared with eyes with intermediate hyperfluorescence (0%).
This study was conducted prospectively in order to determine whether there would be any difference depending on the degree of abnormal choroidal hyperpermeability on ICGA in terms of treatment outcomes, recurrence rates, and complications after half-fluence PDT in patients with chronic CSC.
Materials and methods
This was a prospective observational case series.
Patients Selection
A total of 30 eyes of 30 patients (24 male, six female) diagnosed with chronic CSC were recruited from July 2010 to December 2011 at Yeungnam University Medical Center. In accordance with the guidelines of the Declaration of Helsinki, all subjects provided informed consent for their participation in research. Both the study protocol and informed consent forms were approved by the institutional review board of Yeungnam University.
The inclusion criteria were: (1) persistent symptoms for at least 6 months; (2) presence of subretinal fluid in the foveal region with or without pigment epithelial detachment (PED) on optical coherence tomography (OCT); (3) presence of angiographic leakage on FA; (4) abnormal dilated choroidal vasculature and choroidal vascular hyperpermeability with an area of active leakage on ICGA corresponding to the leaking area on FA.
The exclusion criteria were: (1) age-related maculopathy; (2) angioid streak; (3) pathologic myopia below −6 D; (4) hereditary maculopathy; (5) uveitis; (6) traumatic macular disease; (7) concomitant diabetes or retinal vein occlusion; (8) occurrence of secondary CNV; (9) a medical history of ocular surgery; (10) a history of laser photocoagulation or intravitreal injection.
Classification of baseline ICGA findings
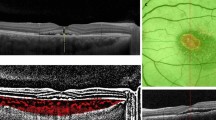
Lesions were classified according to the degree of choroidal hyperfluorescence observed 10 min after injection of indocyanine green.20 When the fluorescence of the primary lesion was much more intense than that of the surrounding area and showed uniform contrast enhancement with a clear boundary, it was identified as intense hyperfluorescence; and when the fluorescence of the primary lesion showed slightly higher contrast enhancement than the surrounding area without a clear boundary, it was identified as weak hyperfluorescence (Figure 1). The intra-observer (two consecutive measurements by MS) and inter-observer (measurements by SHL and MS) agreement for the degree of choroidal hyperfluoresence were assessed by calculating the kappa statistic for the 30 eyes. Each observer, who was masked regarding the patient clinical information, classified each eye into two categories. In cases of disagreement, a third observer (WC) served as an adjudicator.
Classification of ICGA findings at baseline. The ICGA findings before PDT were applied to overlying areas of RPE leakage on FA. ICGA findings during the middle-phase were classified as intense or weak hyperfluorescence. The first row shows intense hyperfluorescence (a, b, c, d) and the second row shows weak hyperfluorescence (e, f, g, h). Middle-phase of FA (a, e), early phase of ICGA (b, f), middle-phase of ICGA (c, g), and late phase of ICGA (d, h).
Treatment and Follow-up
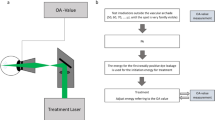
PDT was performed using half of the energy level used in the treatment of AMD with PDT (TAP) protocol;21 6 mg/m2 body surface area of verteporfin (Visudyne, Novartis AG, Basel, Switzerland) was injected intravenously over a period of 10 min; then, 15 min after the start of the injection, the lesion was irradiated with a laser for 83 s at an output of 300 mW (half of the 600 mW output) using a diode laser (Visulas 690 S; Carl Zeiss Meditec Inc., Dublin, CA, USA) capable of delivery at 689 nm light. The area of irradiation included the area of choroidal hyperfluorescence on ICGA corresponding to the leaking points and RPE detachment on FA.
Assessment of patients was performed at baseline and followed up at 1, 2, 3, and 6 months after PDT. The best-corrected visual acuity (BCVA) in Snellen was measured and converted to logarithm of the minimal angle of resolution (logMAR) equivalents. Each 0.10 unit difference in logMAR visual acuity (VA) was considered as one line. OCT images (Stratus OCT; Carl Zeiss Meditec, Inc.) were evaluated at every visit and FA and ICGA (Heidelberg Retinal Angiography II; Heidelberg Engineering, Heidelberg, Germany) were performed before treatment and 3 months after treatment. Additional FA and ICGA were performed in eyes with recurrent subretinal fluid during the follow-up period.
Treatment outcomes and statistical analysis
To assess the efficacy of half-fluence PDT, assessment of visual and tomographic outcomes was performed at every visit during a 6-month follow-up period. Visual outcomes included the change of mean BCVA in logMAR and the proportion of eyes that showed improvement (≥ 2 lines), were stable (within 1 lines), or showed decreased (≥ 2 lines) vision after the initial treatment, when compared with the baseline. Tomographic outcomes included the change of mean central foveal thickness (CFT) and changes in subretinal fluid and PED. Assessment of the rate of recurrence of subretinal fluid and complications was also performed. All patients were classified into groups according to the degree of choroidal hyperpermeability on ICGA at baseline. Treatment outcomes, including visual outcomes, change of mean CFT, tomographic changes, and recurrence of subretinal fluid and complications between the groups were compared. Regarding complications, determination of the severity of choroidal ischemia was based on ICGA performed 3 months after treatment and was analyzed according to the classification suggested by Michels et al.22 Choroidal ischemia was classified according to five grades: Grade 0—No effect on choriocapillaris in early and late ICGA; Grade I—no significant choriocapillary non-perfusion in early ICGA, discrete hypofluorescence in late ICGA; Grade II—Moderate non-perfusion of choriocapillaris in early ICGA; Grade III—Significant non-perfusion of choriocapillaris in early ICGA; Grade IV—Non-perfusion of larger choroidal vessels in early ICGA.
PASW version 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used in performance of statistical analysis. Analysis of changes in BCVA and CFT after PDT was performed in all patients using repeated measures ANOVA to evaluate the efficacy of half-fluence of PDT. For comparison of treatment outcomes according to the degree of choroidal hyperpermeability observed on ICGA, changes in BCVA and CRT between the two groups were compared using repeated measures ANOVA; differences in the recurrence rate between the two groups were compared using Fisher’s exact test. The level of statistical significance was set at P<0.05.
Results
Baseline characteristics of patients focusing on ICGA findings
Subjects included 24 male patients (80.0%) and 6 female patients (20.0%), with a mean age of 47.6±7.5 years (range: 36−65 years). Findings during the middle-phase of ICGA overlying the area of leakage on FA at baseline included intense hyperfluorescence in 16 eyes (53.3%), and weak hyperfluorescence in 14 eyes (46.7%). There were excellent intra-observer and inter-observer agreement in assessing the degree of choroidal hyperfluorescence (kappa=1.000, 0.933). There was no difference in age, gender, duration of symptoms, logMAR BCVA, proportion of macular PED, or PDT spot size at baseline between the two groups (Table 1). However, symptoms of patients in the intense-fluorescence group lasted slightly longer, and the difference in the durations was not statistically significant (P=0.951, Mann–Whitney U-test).
Analysis of the pattern of leakage on FA before PDT was also performed. Of the 16 eyes in the intense-fluorescence group, 12 (75.0%) showed ink-blot leakage patterns, 3 (18.8%) showed ill-defined diffuse leakage patterns, and 1 (6.2%) showed smoke-stack appearance leakage. Of the 14 eyes in the weak-fluorescence group, 6 eyes (42.9%) showed ink-blot patterns, 5 (35.7%) showed ill-defined patterns of diffuse leakage, and 3 (21.4%) showed smoke-stack appearance leakage. No significant difference in leakage patterns on FA was observed between the two groups (P=0.184).
Visual outcomes
Overall, the logMAR BCVA (mean±SD) improved from 0.39±0.21 at baseline to 0.18±0.17 6 months after PDT (P<0.001, repeated measures ANOVA test). At baseline, logMAR BCVA was 0.43 in the intense-hyperfluorescence group and 0.36 in the weak-hyperfluorescence group. After PDT, BCVA showed significant improvement in both the groups (P<0.001, repeated measures ANOVA test). Six months after PDT, mean logMAR BCVA was 0.20 in the intense-hyperfluorescence group and 0.21 in the weak-hyperfluorescence group. The mean BCVA at baseline and 6 months after PDT did not differ significantly between the two groups (P=0.627 and 0.639, Mann–Whitney U-test).
The proportions of eyes showing improved vision of two lines or more was ten eyes (62.5%) in the intense-hyperfluorescence group, and seven eyes (50.0%) in the weak-hyperfluorescence group. The proportion of eyes with stable vision was six eyes (37.5%) in the intense-hyperfluorescence group, and seven eyes (50.0%) in the weak-hyperfluorescence group. No significant difference in proportion of visual acuity improvement (P=0.491, χ2-test) was observed between the two groups. We did not observe any eyes with a decrease in vision in either group during the study period (Figure 2a).
Changes in BCVA and CFT after half-fluence PDT. The proportions of eyes with improved visual acuity and stable visual acuity after 6 months did not differ between the two groups (left, P=0.491, χ2-test). Graph shows a significant reduction in CFT between baseline and at 3 and 6 months (*P<0.001, **P>0.05; Wilcoxon-signed rank test) in each group. However, no significant difference in CFT changes was observed between the two groups during the study period (right).
Tomographic changes
Overall, the mean CFT (mean±SD) showed a significant decrease, from 350.2±80.2 μm at baseline to 198.0±32.6 μm at 6 months after PDT (P<0.001, repeated measures ANOVA test). In the intense-hyperfluorescence group, the CFT showed a significant decrease, from 362.8±86.6 μm at baseline to 196.4±27.0 μm at 6 months after PDT, while in the weak-hyperfluorescence group, the CFT showed a significant decrease, from 335.8±72.6 μm to 199.8±39.2 μm. Nevertheless, no significant difference was observed between the two groups at any time point (P=0.546, repeated measures ANOVA test, Figure 2b). In the intense-fluorescence group, PED was observed in only one eye (6.3%) at baseline. Similarly, in the weak-fluorescence group, PED was observed in only two eyes (14.3%) at baseline. No statistically significant differences were observed (P=1.000). In both the groups, subretinal fluid and PED showed resolution 1 month after treatment.
Recurrence
During the follow-up period, no reaccumulation of subretinal fluid was observed in the intense-fluorescence group. On the other hand, in the weak-hyperfluorescence group, recurrence of subretinal fluid was observed in 1 eye (7.1%) of 14 eyes 6 months after PDT, despite complete resolution at 3 months after PDT. Nevertheless, the rate of recurrence between the two groups did not differ significantly (P=0.467, Fisher’s exact test). The case with reaccumulation of subretinal fluid showed recurred hyperfluorescence at the leakage site identical to the baseline on ICGA 6 months after PDT.
Complications
None of the patients experienced complications related to PDT, including formation of secondary CNV, RPE tear or ripping, RPE atrophy, or subretinal hemorrhage.
On ICGA performed 3 months after PDT, mild choroidal hypoperfusion was observed in three eyes when based on grading by Michels et al.22 Development of choroidal hypoperfusion was observed in two eyes in the intense-hyperfluorescence group (one eye with Grade 1, and one patient with Grade 2), one eye in the weak-hyperfluorescence group (one patient with Grade 1). Nevertheless, the incidence of choroidal hypoperfusion did not differ significantly between the two groups (P=0.467, Fisher’s exact test). Two eyes among them underwent PDT with a relatively large greatest linear dimension of 4024 and 4253 μm.
Discussion
Recently, PDT with verteporfin has been widely used for treatment of CSC, and studies have demonstrated beneficial visual outcomes in most patients.16, 17, 18 Regarding the mechanisms of PDT for treatment of CSC, it is hypothesized that PDT induces selective and transient occlusion of choriocapillaris, which is the source of exudation.23
The true pathophysiology of CSC is not completely understood.24 ICGA for CSC showed disturbance of the choroidal circulation, such as a filling delay in the choroidal arteries and choroidal vascular hyperpermeability, and venous dilation, and suggested that choroidal hyperpermeability might be the origin of CSC.5, 6 In particular, Yannuzzi et al17 introduced ICGA-guided PDT performed as the TAP study on areas of visible hyperpermeability on ICGA. Afterwards, ICGA-guided PDT with application of the TAP protocol was reported to have aided in stabilization of anatomical changes together with the improvement of visual acuity in CSC.15, 19, 20
Some studies have reported that ICGA in eyes with CSC showed diverse intensities of choroidal hyperpermeability overlying the site of fluorescein leakage.1, 9 These findings demonstrate the possibility that treatment outcomes after PDT might vary according to degree of choroidal hyperpermeability regarded as the main pathogenesis of CSC.
Therefore, the authors conducted this study in order to compare and evaluate the efficacy and safety profiles after half-fluence PDT depending on the degree of choroidal hyperfluorescence on ICGA in patients with chronic CSC. In particular, analysis of complications, including choroidal hypoperfusion and recurrence rate of PDT was performed.
In this study, 53.3% of cases (16/30 eyes) showed intense hyperfluorescence in ICGA before PDT, and 46.7% (14/30 eyes) showed weak hyperfluorescence. Thus, a larger number of cases showed intense hyperfluorescence. As this study included only chronic CSC patients who showed ICGA findings on increased permeability of choriocapillaris, dilation of choroidal vessels corresponding to the area of leakage from FA, all of the cases showed choroidal hyperpermeability. These results differ somewhat from those reported by Nicolo et al,19 in which intense hyperfluorescence was 39.4% and weak hyperfluorescence was 60.5%. Inoue et al20 reported that 23 eyes (72%) showed intense hyperfluorescence, 6 eyes (19%) showed weak hyperfluorescence, and 3 eyes (9%) did not show hyperfluorescence. These differences in distribution of choroidal hyperpermeability might be explained by racial characteristics. However, the cause of choroidal hyperpermeability and racial difference was not fully understood.24 Therefore, further study including more patients should be conducted in order to understand distribution of choroidal hyperpermeability.
In the current study, complete subretinal fluid absorption was obtained in 96.6% of eyes (1/30 eyes) at 6-month follow-up after half-fluence PDT. Mean BCVA also showed improvement, from 0.39±0.21 at baseline to 0.18±0.17 at 6 months after PDT. Complete subretinal fluid absorption was obtained in 100% of eyes in the intense-hyperfluorescence group, and only one case (7.1%) with subretinal fluid reaccumulation was observed in the weak-hyperfluorescence group. Changes in mean BCVA and CFT in the intense and weak-hyperfluorescence groups did not differ significantly, as with the rates of subretinal fluid recurrence. Therefore, we could conclude that half-fluence PDT seems to be an effective treatment option for patients with chronic CSC regardless of the degree of hyperfluorescence on ICGA. This may be deemed to indicate the possibility that abnormal hyperpermeability of choroidal blood vessels, as the primary pathogenesis of chronic CSC, may be more important than dysfunction of the RPE. According to recently published data,25 most avascular serous PEDs may represent a variant of CSC in the elderly, and results of PDT for treatment of these lesions were encouraging, despite showing faint choroidal hyperpermeability. Findings of this study supported the suggestion that PDT may be effective regardless of the degree of choroidal hyperpermeability for treatment of CSC.
Inoue et al20 reported that, based on ICGA findings at baseline, 2 eyes (8%) of 23 eyes in the intense-hyperfluorescence group showed recurrence; five eyes (83%) of six eyes with intermediate hyperfluorescence showed recurrence; subretinal fluid did not resolve at 3 months in any eyes in the no hyperfluorescence group (three eyes); recurrence rates of subretinal fluid showed a statistically significant difference between the intense group and intermediate group. In summary, Inoue et al20 claimed that the rate of PDT success is dependent on the degree of choroidal hyperpermeability on ICGA demonstrating more frequent recurrence in eyes with intermediate hyperfluorescence than eyes with intense hyperfluorescence. They hypothesized that in eyes with intermediate hyperfluorescence, a disrupted RPE may not tolerate small changes in hydrostatic pressure, resulting in recurrence besides pre-existing hyperpermeability on ICGA, and they suggested that subretinal fluid in eyes without choroidal hyperfluorescence on ICGA would not result from an imbalance between choroidal hyperpermeability and RPE tolerance but might result from barrier dysfunction of the disrupted RPE.20 However, in their experimental studies, Negi and Marmor26 reported that a simple barrier defect in the RPE did not cause retinal detachment; these findings are contrary to the hypothesis of Inoue et al. In addition, Nicolo et al19 suggested that resolution of exudation might be expected in eyes with subretinal fluid regardless of the intensity of choroidal hyperfluorescence after half-dose PDT; these findings were compatible with our results. Although they reported an association between the efficacy of PDT with ICGA and OCT, the study had major limitation of retrospective study design.19
Standard PDT based on the conventional TAP protocol frequently resulted in secondary complications, such as choriocapillary ischemia after 12 months, secondary RPE changes, or CNV formation related to hypoxic damage.7, 8, 9, 10, 23, 27 In order to reduce such complications, half-dose or half-fluence PDT has been attempted by different authors, who reported that it is effective enough to induce reabsorption of subretinal fluid with some beneficial visual outcomes in most patients.18, 28
In particular, to the best of our knowledge, the current study is the first report to compare choroidal ischemia after half-fluence PDT according to baseline choroidal hyperpermeablity in chronic CSC. During the 6-month follow-up period, three cases of choroidal ischemia were noted: two in the intense-hyperfluorescence group and one in the weak-hyperfluorescence group. Thus, no difference according to the degree of choroidal hyperfluorescence assessed using ICGA was observed (P=0.467). The two eyes showed mild (Grade 1) choroidal hypoperfusion and only one eye showed moderate (Grade 2) choroidal hypoperfusion. None of the eyes with choroidal hypoperfusion showed a decrease in vision during the study period.
Reibaldi et al18 reported similar results in the 1-year follow-up. They reported observance of a moderate-significant choriocapillaris non-perfusion in 8 of 18 standard fluence-treated eyes and 0 of 23 low-fluence-treated eyes (44% vs 0%; P=0.002). The effect of blood flow impairment in the normal choroid after PDT on visual acuity was not elucidated.
Michaels et al22 reported that, among patients who showed mild choroidal non-perfusion at the time of their follow-up three months after PDT, none experienced deterioration of visual acuity by more than two lines, and the difference in normal choroidal perfusion showed no association with the change in initial visual acuity. However, in the TAP study21 and the VIP study (verteporfin in the PDT),29 after PDT, rapid deterioration of visual acuity was observed in 0.7–4.9% of cases due to subretinal hemorrhage, serous retinal detachment, and choroidal ischemia. In particular, the better the visual acuity was (higher than 20/50), the more severe the deterioration of visual acuity was noted in cases with choroidal ischemia. Thus, it is suggested that, when performing PDT, greater attention should be paid to chronic CSC patients with relative good visual acuity in comparison with other macular diseases.
Although this study has the strength of being a prospective study, there are some weaknesses, including the small sample size and relatively short-term follow-up design considering the disease course of chronic CSC. Therefore, further study is needed in order to determine whether there would be any difference in treatment outcomes and recurrence rates in long-term follow-up after PDT according to the degree of choroidal hyperfluorescence on ICGA.
In conclusion, findings of this study indicated that ICGA-guided half-fluence PDT seems to be an effective and safe treatment option for patients with long-standing chronic CSC. In addition, treatment outcomes did not differ significantly between intense and weak-hyperfluorescence groups, as with the subretinal recurrence rates; these findings suggest that choroidal hyperpermeability might be more important than dysfunction of RPE as the primary pathogenesis of chronic CSC.

References
Piccolino FC, Borgia L, Zinicola E, Zingirian M . Indocyanine green angiographic findings in central serous chorioretinopathy. Eye 1995; 9: 324–332.
Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996; 103: 2070–2079 (discussion 2079–2080).
Gemenetzi M, De Salvo G, Lotery AJ . Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye 2010; 24: 1743–1756.
Spitznas M . Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol 1986; 224: 321–324.
Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina 1996; 16: 203–213.
Prunte C, Flammer J . Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 1996; 121: 26–34.
Jalkh AE, Jabbour N, Avila MP, Trempe CL, Schepens CL . Retinal pigment epithelium decompensation. I. Clinical features and natural course. Ophthalmology 1984; 91: 1544–1548.
Ie D, Yannuzzi LA, Spaide RF, Rabb MF, Blair NP, Daily MJ . Subretinal exudative deposits in central serous chorioretinopathy. Br J Ophthalmol 1993; 77: 349–353.
Iida T, Yannuzzi LA, Spaide RF, Borodoker N, Carvalho CA, Negrao S . Cystoid macular degeneration in chronic central serous chorioretinopathy. Retina 2003; 23: 1–7 (quiz 137–138).
Ficker L, Vafidis G, While A, Leaver P . Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1988; 72: 829–834.
Piccolino FC . Laser treatment of eccentric leaks in central serous chorioretinopathy resulting in disappearance of untreated juxtafoveal leaks. Retina 1992; 12: 96–102.
Lim SJ, Roh MI, Kwon OW . Intravitreal bevacizumab injection for central serous chorioretinopathy. Retina 2010; 30: 100–106.
Schaal KB, Hoeh AE, Scheuerle A, Schuett F, Dithmar S . Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol 2009; 19: 613–617.
Huang WC, Chen WL, Tsai YY, Chiang CC, Lin JM . Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eye 2009; 23: 488–489.
Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM . Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003; 23: 752–763.
Chan WM, Lam DS, Lai TY, Tam BS, Liu DT, Chan CK . Choroidal vascular remodelling in central serous chorioretinopathy after indocyanine green guided photodynamic therapy with verteporfin: a novel treatment at the primary disease level. Br J Ophthalmol 2003; 87: 1453–1458.
Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa DL, Huang SJ et al. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2003; 23: 288–298.
Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S et al. Standard-fluence versus low-fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. Am J Ophthalmol 2010; 149: 307–315.
Nicolo M, Zoli D, Musolino M, Traverso CE . Association between the efficacy of half-dose photodynamic therapy with indocyanine green angiography and optical coherence tomography findings in the treatment of central serous chorioretinopathy. Am J Ophthalmol 2012; 153: 474–480.
Inoue R, Sawa M, Tsujikawa M, Gomi F . Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol 2010; 149: 441–446.
Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials--TAP report. Arch Ophthalmol 1999; 117: 1329–1345.
Michels S, Hansmann F, Geitzenauer W, Schmidt-Erfurth U . Influence of treatment parameters on selectivity of verteporfin therapy. Invest Ophthalmol Vis Sci 2006; 47: 371–376.
Schlotzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U . Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol 2002; 240: 748–757.
Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T . Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina 2011; 31: 1603–1608.
Kim KS, Lee WK . Photodynamic therapy with verteporfin for avascular serous pigment epithelial detachment in elderly Koreans. Retina 2010; 30: 93–99.
Negi A, Marmor MF . Experimental serous retinal detachment and focal pigment epithelial damage. Arch Ophthalmol 1984; 102: 445–449.
Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, Viestenz A, Naumann GO . Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol 2002; 120: 835–844.
Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS . Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina 2008; 28: 85–93.
Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalmol 2001; 131: 541–560.
Acknowledgements
Publication of this article was supported by a Yeungnam University research grant in 2011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lim, S., Chang, W. & Sagong, M. Efficacy of half-fluence photodynamic therapy depending on the degree of choroidal hyperpermeability in chronic central serous chorioretinopathy. Eye 27, 353–362 (2013). https://doi.org/10.1038/eye.2013.13
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.13
Keywords
This article is cited by
-
No-Dose Photodynamic Therapy Against Half-Dose Photodynamic Therapy for Treatment of Central Serous Chorioretinopathy
Ophthalmology and Therapy (2023)
-
Long-term results of focal laser photocoagulation and photodynamic therapy for the treatment of central serous chorioretinopathy
Japanese Journal of Ophthalmology (2020)
-
The comparison of multimodal imaging findings of central serous chorioretinopathy patients in regard to the early anatomically treatment response to half-fluence photodynamic therapy: a retrospective case–control study
International Journal of Retina and Vitreous (2017)
-
Leitlinien für die Betreuung und Behandlung von Patienten mit Chorioretinopathia centralis serosa
Spektrum der Augenheilkunde (2016)
-
Oral Rifampin treatment for longstanding chronic central serous chorioretinopathy
Graefe's Archive for Clinical and Experimental Ophthalmology (2016)