Abstract
Purpose
To compare the safety and efficacy of two polypropylene (Prolene) sutures for tensioning of the inner wall of Schlemm's canal (SC) in black African patients with primary open-angle glaucoma (POAG) undergoing canaloplasty.
Methods
In a prospective randomised trial of 90 patients, canaloplasty was performed with a flexible microcatheter (iTrack-250A) and sodium hyaluronidate 1.4% (Healon GV). After complete circumferential dilatation of the SC, a Prolene suture, either 6–0 Prolene (group 1) or 10–0 Prolene (group 2), was retracted through the SC and tightened leaving tension on the canal and trabecular meshwork. Nd:YAG laser goniopuncture was not performed postoperatively.
Results
The mean preoperative intraocular pressure (IOP) was 42.7 mm Hg±12.5 (SD) in group 1 and 45.0 mm Hg±12.1 (SD) in group 2 (P=0.70). The mean postoperative IOP without medications was 18.4 mm Hg±7.1 (SD) in group 1 and 16.4 mm Hg±6.6 (SD) in group 2 at 1 month (P=0.10), 19.2 mm Hg±6.4 (SD) in group 1 and 16.4 mm Hg±4.9 (SD) at 15 months (P=0.04). Pressures equal or less than 21, 18, and 16 mm Hg without medications (complete success) at 12 months were 51.0% (95% confidence interval (CI) 0.35–0.73), 34.1% (95% CI 0.21–0.56), and 21.2% (95% CI 0.11–0.42) in group 1, and 76.9% (95% CI 0.62–0.96), 68.8% (95% CI 0.54–0.89), and 53.6% (95% CI 0.38–0.76) in group 2, respectively. In the Cox regression analysis, IOP <18 mm Hg without medications depended significantly on the type of Prolene (hazard ratio (HR) 2.60, 95% CI 1.24–5.46, P=0.01) and age (HR 1.3, 95% CI 1.03–1.86, P=0.03), but not on preoperative IOP (HR 1.01, 95% CI 0.99–1.04, P=0.16) and gender (HR 0.67, 95% CI 0.34–1.33, P=0.26). No filtering bleb was observed. Intra- and postoperative complications were similarly rare in the two groups and included partial ‘cheese-wiring’ (2), Descemet's rupture (2), and hyphaema (3).
Conclusions
In this clinical trial, IOP reduction was substantial in canaloplasty and slightly greater in combination with 10–0 Prolene than 6–0 Prolene sutures at an equally low complication rate. Younger age, but not the level of IOP at surgery, had a positive effect on the amount of IOP reduction, thus suggesting that an early surgical intervention to re-establish physiological outflow offers the best prognosis.
Similar content being viewed by others
Introduction
Classic glaucoma surgery, such as trabeculectomy and shunts, lowers intraocular pressure (IOP) by redirecting aqueous humour outflow into the subconjunctival space (external filtration). They are proven to be effective in reducing IOP; however, the intra-and postoperative complications are numerous.1, 2, 3 Complications are often related to bleb such as leaks, scarring, and infections (blebitis, endophthalmitis), or to hypotony-like shallow anterior chamber, choroidal effusion, cataract, and maculopathy. The prevention of such complications has led to the concept of non-penetrating glaucoma surgery. Excising sclero-corneal stroma over Descemet's membrane (DM) and anterior trabeculum results in a thin DM window providing some resistance to aqueous outflow. This strategy is called deep sclerectomy and was first described by Fyodorov et al4 and Kozlov et al.5 Since then, many modifications with and without implants have been reported. The idea behind non-penetrating procedures is also to surgically restore the natural aqueous outflow, rather than to create a new and possibly excessively potent drainage site. In 1995, viscocanalostomy was described as a bleb-independent procedure,6 in which Schlemm's canal (SC) is expanded with visco-elastic material. Aqueous outflow is postulated to pass through the DM to the scleral lake and to follow the physiological pathway distal to the dilated SC without external filtration. Direct comparison to trabeculectomy yielded a lower incidence of postoperative complications,7, 8 but IOP reduction was found to be less effective.9, 10
Viscocanalostomy is able to open just 120° of SC, limiting its effectiveness and enhancing the procedure's likelihood of failure; as far as our experience goes, re-collapse of SC and the closure of the ostia of the collector channels are the main causes for failure in viscocanalostomy. Enlarging a greater part may increase the chance for a more consistent patency of SC and a circumferential flow.11 The idea of re-establishing the internal physiological pathway was the initiation of the development of a lighted flexible microcatheter (iTrack; iScience Interventional, Menlo Park, CA, USA). In a study on human perfused cadaver eyes, 180° expansion of SC showed a significant increase in the outflow facility and amount of expansion was related to the relative increase in outflow facility.12 More recently, viscodilation of the entire SC demonstrated a promising reduction in IOP with few postoperative complications in a clinical study (‘enhanced’ viscocanalostomy).13 A 360° tensioning suture placed in the canal has led to the technique that is called canaloplasty.14 The rationale for 360° suture distension in canaloplasty is to maintain increased permeability of the inner wall region, to ensure circumferential flow and to reduce the risk of re-collapse. To date, all studies published on canaloplasty used 10–0 (non-absorbable, polypropylene) Prolene suture to distend the inner wall of SC.14, 15, 16 However, it is questionable whether 10–0 Prolene suture is the ideal candidate for canal distension because of its thin diameter and the potential risk of cheese-wiring. In this study, we sought to compare the safety and efficacy of 10–0 Prolene vs 6–0 Prolene suture in patients with primary open-angle glaucoma (POAG) undergoing canaloplasty.
Materials and methods
Setting
This prospective study was performed at the Department of Ophthalmology, Medical University of Southern Africa, Medunsa, South Africa, and was approved by the institutional ethics committees. Informed consent was obtained from each patient. The research followed the tenets of the Declaration of Helsinki.
Study population
A total of 90 consecutive black African patients with POAG scheduled for canaloplasty were prospectively enrolled for this study. The following were not included in the study: eyes of patients with narrow or closed iridocorneal angle; evidence for any secondary glaucoma; pigmentary dispersion; pseudoexfoliation; a history of trauma, of chronic or recurrent inflammatory eye disease (eg scleritis, uveiitis); and with any type of preceding refractive surgery and corneal disease. Patients with POAG were randomly assigned to receive either a 6–0 or 10–0 Prolene suture. Diurnal IOP measurements the day before surgery were taken and the mean IOP was used for statistical analysis.
Surgical technique
After limbal peritomy, a superficial and deep scleral flap was dissected as previously described for viscocanalostomy.6 The superficial flap had a parabolic shape (about 5.0 mm × 5.0 mm) and of about one-third scleral thickness to ensure water-tight closure at the end of surgery. The second flap constituted approximately two-thirds of the scleral thickness to leave a thin translucent layer of sclera overlying the choroid. In this plane, the deep flap was dissected to the limbal area until the SC was unroofed. Both ostia of the SC were dissected forward to expose the DM. A flexible microcatheter (iTrack-250A; iScience) was introduced into the ostia of SC and advanced 360° to dilate stepwise the canal by injecting microvolumes of sodium hyaluronidate 1.4% (Healon GV). After complete circumferential dilatation, a Prolene suture (6–0 or 10–0) was affixed to the distal tip of the microcatheter and looped through the canal. The suture was tightened to stretch the inner wall of SC and the TM circumferentially. The distension of the canal was observed clinically by the inward movement of the suture and inner wall of exposed SC. The suture was tightened under tension on a soft eye in order to stretch the SC and trabecular meshwork circumferentially when IOP returned to normal. The deep scleral flap was excised and the superficial flap was closed and sutured watertight.
Postoperative follow-up
The IOP was measured before surgery and 1 week, 1, 3, 6, 9, 12, and 15 months, then 3–6 monthly thereafter. Postoperative treatment was standardised and included antibiotic, steroid, and non-steroid eye drops four times a day for the first month and three times a day for the second month. Nd:YAG laser goniopuncture was not performed postoperatively for study purposes, as it may conceal the true action of canaloplasty by its IOP lowering effect.
Statistical analysis
Frequency tables were used to describe categorical variables and descriptive statistics to determine continuous variables (demographic data). Complete success was defined as an IOP 21 mm Hg or lower without medications and qualified with or without medications, respectively. The data were also analysed with regard to target pressure of <18 mm Hg in agreement with the Advanced Glaucoma Intervention Study (AGIS),17 of <16 mm Hg according to the recommendation of the World Glaucoma Association and to a 30% IOP reduction, as calculated for a reduced relative risk of progression.18, 19 Owing to the high IOP levels before surgery, the surgical success was also analysed with regard to a 50% IOP reduction. In addition, a Kaplan–Meier survival analysis was used to determine the cumulative probability of an IOP greater than 21, 18, and 16 mm Hg in either group, beginning at week 1 after surgery to ensure that the visco-elastic was washed out and does not influence IOP. The endpoint was the time of first confirmation of the presence of IOP more than 21, 18, and 16 mm Hg. The difference in terms of reaching cut-off 21, 18, and 16 mm Hg between Prolene sutures was analysed using the log rank-test between the Kaplan–Meier probability curves. In addition, a Cox proportional hazard analysis was carried out to evaluate the factors potentially associated with the failure of IOP reduction below 18 mm Hg including preoperative IOP, age at surgery, gender, and type of Prolene suture. Corresponding hazard ratios (HRs) with 95% confidence interval (CI) were estimated. HRs for continuous factors such as IOP and age were defined as the ratio of an increase in IOP of 1 mm Hg, and an increase in age of 10 years, respectively. Assumption of proportional hazard20 was carefully assessed for each independent factor. Statistical analyses were carried out using SPSS software version 13.0 (SPSS, Chicago, IL, USA). A P-value of <0.05 was considered statistically significant. Other outcome parameters of interest were intra- and postoperative complications.
Results
The mean age (43.5 years±21.4 (SD) in group 1 (6–0 Prolene)), and 46.8 years±21.9 (SD) in group 2 (10–0 Prolene); P=0.95), the mean follow-up time (8.7 months±3.9 (SD) in group 1, and 10.4 months±5.3 (SD) in group 2; P=0.11), the mean preoperative IOP (42.7±12.5 (SD) in group 1; 45.0 mm Hg±12.1 (SD) in group 2; P=0.70) and the mean cup : disc ratio (0.78±0.42 (SD) in group 1; 0.81±0.38 (SD) in group 2; P=0.82) were comparable between the two groups.
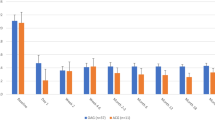
In group 1, the mean postoperative IOP was 18.4 mm Hg±7.1 (SD) 1 month after surgery, 18.3 mm Hg±6.2 (SD) at 3 months, 18.3 mm Hg±6.2 (SD) at 6 months, 19.1 mm Hg±7.4 (SD) at 12 months, and 19.2 Hg±6.4 (SD) at 15 months. In group 2, the mean postoperative IOP was 16.4 mm Hg±6.6 (SD) 1 month after surgery, 16.7 mm Hg±5.9 (SD) at 3 months, 16.1 mm Hg±5.6 (SD) at 6 months, 16.6 mm Hg±5.3 (SD) at 9 months, 16.9 mm Hg±4.8 (SD) at 12 months, and 16.4 mm Hg±4.9 (SD) at 15 months (Table 1 and Figure 1).
At 15 months after surgery, IOP reduction of 30% without medications was achieved in 96.8% of group 1, and in 97.8% of group 2, and of 50% reduction of IOP was 55.6% in group 1, and 83.9% in group 2, respectively. The differences in the complete success rates of an IOP of <21, <18 and <16 mm Hg were more significant between the two groups the lower the target IOP was defined (Kaplan–Meier survival curves; Figure 2a and b). The cumulative probability of success for an IOP of 21, 18, or 16 mm Hg, or less after 15 months was 51.0% (95% CI 0.35–0.73), 34.1% (95% CI 0.21–0.56), and 21.2% (95% CI 0.11–0.42) in group 1, and 76.9% (95% CI 0.62–0.96), 68.8% (95% CI 0.54–0.89), and 53.6% (95% CI 0.38–0.76) in group 2, respectively. In the Cox regression analysis, complete success rate (IOP <18 mm Hg without medications) depended significantly on the type of Prolene (HR 2.60, 95% CI 1.24–5.46, P=0.01), and age (HR 1.3, 95% CI 1.03–1.86, P=0.03), but not on preoperative IOP (HR 1.01, 95% CI 0.99–1.04, P=0.16) and gender (HR 0.67, 95% CI 0.34–1.33, P=0.26).
Intra- and postoperative complications were rare in both the groups (Table 2) and included partial ‘cheese-wiring’ (2), Descemet's rupture (2), macrohyphaema (3), hypotony (0), choroidal detachment (0), and so on. Microhyphaema of <2 mm was a common observation after canaloplasty.
Discussion
Canaloplasty is a new, non-penetrating approach in glaucoma surgery, which intends to re-establish the internal physiological pathway by overcoming the site of maximal resistance to aqueous outflow in POAG by distension of the inner wall region with a circumferential tensioning suture. Previous studies on canaloplasty used exclusively 10–0 Prolene suture for tensioning the inner wall of SC.14, 15, 16 This study is the first of its kind comparing two different suture sizes for 360° tensioning of SC. In this series, mean IOP reduction was greater in combination with 10–0 Prolene than 6–0 Prolene sutures at each time point during the study period. Further, patient's age, but not the pressure level before surgery was decisive for the amount of IOP reduction.
The average postoperative IOP was 16.1 mm Hg at 6 months and 16.9 mm Hg at 12 months in the 10–0 Prolene group slightly higher than previously reported (15.3 and 15.6 mm Hg, respectively).14 A reason for this IOP difference may be different study populations with different stages of glaucoma. In the multi-centre study, the great majority of patients were Caucasians (89.8%) with moderate glaucomatous damage and mean IOP of around 25 mm Hg,14 whereas in this study, all patients were black Africans with advanced POAG and mean IOP of around 45 mm Hg. The postoperative IOP was on average 2 mm Hg lower in the 10–0 than in the 6–0 Prolene group. When stronger criteria were applied to define complete success (without medications), such as an IOP below 18 mm Hg17or 16 mm Hg, it became more evident that IOP reduction was consistently lower in the 10–0 Prolene than in the 6–0 Prolene group (68.8 vs 34.1% and 53.6 vs 21.2%, respectively). Better distension of the canal by the 10–0 Prolene, or partial obstruction of the TM by the 6–0 Prolene because of its large diameter size, or both may have contributed to the more persistent IOP reduction in the 10–0 Prolene group. To date, the 6–0 Prolene thread has been commonly used for 360° suture trabeculotomy21, 22, 23 or as a tool for suture retraction in catheter-less canaloplasty,24 but the role as a canal stent is currently unknown.
The postoperative IOP in the 10–0 Prolene group was similar to the mean IOP of a recent meta-analysis of viscocanalostomy (16.4 mm Hg; range 13–20 mm Hg),25 and possibly less than one might expect from the additional step of the tensioning suture in canaloplasty. However, the rate of goniopuncture in the reported studies was between 9 and 56%.7, 8, 26, 27 It is well-known that laser goniopuncture produces lower IOP,7, 27 but also converts a non-penetrating designed procedure6 into a perforating one. Sharaawy et al27 reported a 38.2% drop in IOP from 20.4 mm Hg before to 12.6 mm Hg after goniopuncture. In this series, goniopuncture was not carried for the purpose of studying the genuine effect of canaloplasty. At present, we do not know whether canaloplasty may have an effect in reducing the need for goniopuncture by canal distension in the long run as the follow-up period was 15-months. Possibly, lower IOP would have been achieved by means of goniopuncture and some patients may require goniopuncture later on, as it is known that a significant proportion of patients who have had non-penetrating glaucoma surgery required goniopuncture in the subsequent years.
Various surgeons sutured the superficial flap loosely in viscocanalostomy with the intention of bleb formation8, 26, 27 in contrast to Stegmann's original description.6 Even upto 35% of eyes had undergone 5-fluorouracil injection after viscocanalostomy to control IOP.8, 27, 28 In studies that report on the presence of a filtration bleb and goniopuncture, postoperative IOP levels were low between 13.0 and 15.5 mm Hg.26, 27, 29 If no such additional procedures were performed, the mean IOP was correspondingly higher (upto 17.8 mm Hg).9, 30, 31 Although we acknowledge that subconjunctival filtration is one of the methods of drainage, 5-fluorouracil injection is helpful to control wound healing, and laser goniopuncture increases aqueous outflow, the diversity of surgical modifications and postoperative manipulations makes direct comparison of efficacy difficult. From a pathophysiological perspective, the term ‘non-penetrating’ glaucoma surgery is imprecise and should be reconsidered. It might be more appropriate to distinguish between bleb-independent and bleb-dependent glaucoma surgery.
Older age was predictive for failure of canaloplasty in this study. Recent studies on canaloplasty have not considered age into their analyses.14, 15, 16 Alteration of the TM along with atrophy of the distal system in case of herniation of the wall into the collector channels ostia32, 33, 34 could have contributed besides a physiological aging process of inner wall.35 In contrast with the age factor, IOP level before surgery did not seem to have an effect on IOP reduction as canaloplasty may directly target the major site of outflow resistance. The inconsistent dependence of IOP reduction on age and preoperative IOP, found here, suggests that after restoring the proximal outflow system (ie, TM and SC), the distal system (ie, collector channels and episcleral venous system) may still be fully patent in the young but not in the elderly glaucoma patient.
The complication rate is inherently low in canaloplasty,14, 15 as it is not only a non-penetrating but also a bleb-independent procedure. This is also reflected in this study. Bleb-associated problems and hypotony are virtually non-existent. Suture ‘cheese wiring’ into the anterior chamber during and after surgery was not a frequent complication in canaloplasty, possibly resulting from the underestimated tensile strength of the TM. We did not find any difference in the frequency of cheese-wiring between the 6–0 and 10–0 Prolene group, thereby indicating that neither the thickness nor the elasticity of Prolene sutures36, 37 may be relevant for this adverse event.
In conclusion, canaloplasty was an effective method to lower IOP in patients with POAG. The use of 10–0 Prolene seems to enhance the success rate and resulted in a lower IOP than with 6–0 Prolene, possibly because of better distension of the canal. Further, patients of younger age at surgery had a greater benefit from canaloplasty than elderly patients in this series, possibly owing to the functional state of the distal outflow system.

References
Jones E, Clarke J, Khaw PT . Recent advances in trabeculectomy technique. Curr Opin Ophthalmol 2005; 16: 107–113.
Mac I, Soltau JB . Glaucoma-filtering bleb infections. Curr Opin Ophthalmol 2003; 14: 91–94.
Borisuth NS, Phillips B, Krupin T . The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol 1999; 10: 112–116.
Kozlov V, Bagrov SN, Anisimova SY . Nonpenetrating deep sclerectomy with collagen. Eye Microsurgery 1990; 3: 44–46.
Fyodorov SID, Ronkina TI . Glaucoma surgery – deep sclerectomy. Vestn Oftalmol 1982; 4: 6–10.
Stegmann R . Visco-canalostomy: a new surgical technique for open angle glaucoma. An Inst Barraquer, Spain 1995; 25: 229–232.
Kobayashi H, Kobayashi K, Okinami S . A comparison of the intraocular pressure-l owering effect and safety of viscocanalostomy and trabeculectomy with mitomycin C in bilateral open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 2003; 241: 359–366.
O’Brart DP, Shiew M, Edmunds B . A randomised, prospective study comparing trabeculectomy with viscocanalostomy with adjunctive antimetabolite usage for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol 2004; 88: 1012–1017.
Yalvac IS, Sahin M, Eksioglu U, Midillioglu IK, Aslan BS, Duman S . Primary viscocanalostomy vs trabeculectomy for primary open-angle glaucoma: three-year prospective randomized clinical trial. J Cataract Refract Surg 2004; 30: 2050–2057.
Carassa RG, Bettin P, Fiori M, Brancato R . Viscocanalostomy vs trabeculectomy in white adults affected by open-angle glaucoma: a 2-year randomized, controlled trial. Ophthalmology 2003; 110: 882–887.
Smit BA, Johnstone MA . Effects of viscoelastic injection into Schlemm′s canal in primate and human eyes: potential relevance to viscocanalostomy. Ophthalmology 2002; 109: 786–792.
Hee M, Conston S, Yamamoto R . Outflow facility after 180 degree catheterization and distal expansion of Schlemm′s canal perfused cadaver eyes [abstract]. ARVO 2004; Poster # 984
Cameron B FM, Ball S, Kearney J . Circumferential viscodilation of Schlemm′s canal with a flexile microcannula during non-penetrating glaucoma surgery. Digit J Ophthalmol 2006; 12 (1) Available at: http://www.djo.harvard.edu/site.php?url=/physicians/oa/929.
Lewis RA, von Wolff K, Tetz M, Korber N, Kearney JR, Shingleton B et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm′s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg 2007; 33: 1217–1226.
Lewis RA, von Wolff K, Tetz M, Korber N, Kearney JR, Shingleton B et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg 2009; 35: 814–824.
Shingleton B, Tetz M, Korber N . Circumferential viscodilation and tensioning of Schlemm canal (canaloplasty) with temporal clear corneal phacoemulsification cataract surgery for open-angle glaucoma and visually significant cataract: one-year results. J Cataract Refract Surg 2008; 34: 433–440.
The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS investigators. Am J Ophthalmol 2000; 130: 429–440.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M . Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120: 1268–1279.
The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol 1998; 126: 498–505.
Therneau TM, Grambsch PM . Modeling Survival Data. Springer Verlag: Berlin, 2000.
Beck AD, Lynch MG . 360 degrees trabeculotomy for primary congenital glaucoma. Arch Ophthalmol 1995; 113: 1200–1202.
Smith R . A new technique for opening the canal of Schlemm. Preliminary report. Br J Ophthalmol 1960; 44: 370–373.
Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy vs goniotomy. J AAPOS 2000; 4: 205–210.
Scharioth G . Catheterless canaloplasty. In: Scharrer A, Neuhann T (eds). Fortschritte der Ophthalmochirurgie 2008. Aktiv Druck& Verlag GmbH: Nürnberg, 2008; p 91.
Hondur A, Onol M, Hasanreisoglu B . Nonpenetrating glaucoma surgery: meta- analysis of recent results. J Glaucoma 2008; 17 (2): 139–146.
Sunaric-Megevand G, Leuenberger PM . Results of viscocanalostomy for primary open-angle glaucoma. Am J Ophthalmol 2001; 132 (2): 221–228.
Shaarawy T, Nguyen C, Schnyder C, Mermoud A . Five-year results of viscocanalostomy. Br J Ophthalmol 2003; 87 (4): 441–445.
O’Brart DP, Rowlands E, Islam N, Noury AM . A randomised, prospective study comparing trabeculectomy augmented with antimetabolites with a viscocanalostomy technique for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol 2002; 86 (7): 748–754.
Yarangumeli A, Koz OG, Alp MN, Elhan AH, Kural G . Viscocanalostomy with mitomycin-C: a preliminary study. Eur J Ophthalmol 2005; 15 (2): 202–208.
Wishart PK, Wishart MS, Choudhary A, Grierson I . Long-term results of viscocanalostomy in pseudoexfoliative and primary open angle glaucoma. Clin Experiment Ophthalmol 2008; 36 (2): 148–155.
Drusedau MU, von Wolff K, Bull H, von Barsewisch B . Viscocanalostomy for primary open-angle glaucoma: the Gross Pankow experience. J Cataract Refract Surg 2000; 26 (9): 1367–1373.
Johnstone MA, Grant WG . Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol 1973; 75: 365–383.
Allingham RR, de Kater AW, Ethier CR . Schlemm's canal and primary open angle glaucoma: correlation between Schlemm′s canal dimensions and outflow facility. Exp Eye Res 1996; 62: 101–109.
Nesterov AP . Role of the blockade of Schlemm's canal in pathogenesis of primary open-angle glaucoma. Am J Ophthalmol 1970; 70: 691–696.
Boldea RC, Roy S, Mermoud A . Ageing of Schlemm′s canal in nonglaucomatous subjects. Int Ophthalmol 2001; 24: 67–77.
O’Driscoll AM, Goble RR, Hallack GN, Andrew NC . A prospective, controlled study of a 9/0 elastic Prolene suture for cataract surgery: refractive results and complications. Eye (London, England) 1994; 8 (Pt 5): 538–542.
O’Driscoll AM, Quraishy MM, Andrew NC . Elastic Prolene suture in cataract surgery: long-term follow-up. Eye (London, England) 1996; 10 (Pt 1): 99–102.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Stegmann has received an unrestricted grant from iScience Interventional. No other author has received public or private financial support nor has a proprietary or financial interest in any material or method mentioned.
Rights and permissions
About this article
Cite this article
Grieshaber, M., Pienaar, A., Olivier, J. et al. Comparing two tensioning suture sizes for 360° viscocanalostomy (canaloplasty): a randomised controlled trial. Eye 24, 1220–1226 (2010). https://doi.org/10.1038/eye.2009.317
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2009.317
Keywords
This article is cited by
-
Modified suture-assisted canaloplasty in Asians with primary open-angle glaucoma: a prospective study with 12-month follow-up
BMC Ophthalmology (2022)
-
Combined Ab interno viscocanaloplasty (ABiC) in open-angle glaucoma: 12-month outcomes
International Ophthalmology (2021)
-
Outcomes of 360° suture trabeculotomy after unsuccessful canaloplasty
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)
-
Fluorescein channelography in canaloplasty: quantitative approach
Spektrum der Augenheilkunde (2016)
-
Exit strategies in canaloplasty: intraoperative conversion into 180-degree trabeculotomy or 360-degree trabeculotomy in cases of unsuccessful catheterisation of Schlemm’s canal: influence of degree of canal cleavage
Graefe's Archive for Clinical and Experimental Ophthalmology (2015)