Abstract
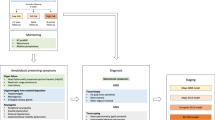
Optimal treatment approach continues to remain a challenge for systemic light chain amyloidosis (AL). So far, Auto-SCT is the only modality associated with long-term survival. However, failure to show survival benefit in randomized study raises questions regarding its efficacy. We present a comparative outcome analysis of Auto-SCT to conventional therapies (CTR) in AL patients treated over a 14-year period at our institution. Out of the 145 AL amyloidosis patients, Auto-SCT was performed in 80 patients with 1-year non-relapse mortality rate of 12.5%. Novel agents were used as part of induction therapy in 56% of transplant recipients vs 46% of CTR patients. Hematological and organ responses were seen in 74.6% and 39% in the Auto-SCT arm vs 53% and 12% in the CTR arm, respectively. The projected 5-year survival for Auto-SCT vs CTR was 63% vs 38%, respectively. Landmark analysis of patients alive at 1-year after diagnosis showed improved 5-year OS of 72% with Auto-SCT vs 65% in the CTR arm. In the multivariate analysis, age <60 years, induction therapy with novel agents, kidney only involvement and Auto-SCT were associated with improved survival. In conclusion, Auto-SCT is associated with long-term survival for patients with AL amyloidosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Merlini G, Seldin DC, Gertz MA . Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol 2011; 29: 1924–1933.
Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med 1997; 336: 1202–1207.
Gertz MA . I don't know how to treat amyloidosis. Blood 2010; 116: 507–508.
Palladini G, Perfetti V, Obici L, Caccialanza R, Semino A, Adami F et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood 2004; 103: 2936–2938.
Palladini G, Perfetti V, Perlini S, Obici L, Lavatelli F, Caccialanza R et al. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL). Blood 2005; 105: 2949–2951.
Wechalekar AD, Goodman HJ, Lachmann HJ, Offer M, Hawkins PN, Gillmore JD . Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood 2007; 109: 457–464.
Dispenzieri A, Lacy MQ, Zeldenrust SR, Hayman SR, Kumar SK, Geyer SM et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood 2007; 109: 465–470.
Sanchorawala V, Wright DG, Rosenzweig M, Finn KT, Fennessey S, Zeldis JB et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood 2007; 109: 492–496.
Dimopoulos MA, Kastritis E . Bortezomib for AL amyloidosis: moving forward. Blood 2011; 118: 827–828.
Sanchorawala V, Quillen K, Sloan JM, Andrea NT, Seldin DC . Bortezomib and high-dose melphalan conditioning for stem cell transplantation for AL amyloidosis: a pilot study. Haematologica 2011; 96: 1890–1892.
Sanchorawala V, Seldin DC, Magnani B, Skinner M, Wright DG . Serum free light-chain responses after high-dose intravenous melphalan and autologous stem cell transplantation for AL (primary) amyloidosis. Bone Marrow Transplant 2005; 36: 597–600.
Perz JB, Schonland SO, Hundemer M, Kristen AV, Dengler TJ, Zeier M et al. High-dose melphalan with autologous stem cell transplantation after VAD induction chemotherapy for treatment of amyloid light chain amyloidosis: a single centre prospective phase II study. Br J Haematol 2004; 127: 543–551.
Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med 2004; 140: 85–93.
Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med 2007; 357: 1083–1093.
Sanchorawala V, Wright DG, Seldin DC, Dember LM, Finn K, Falk RH et al. An overview of the use of high-dose melphalan with autologous stem cell transplantation for the treatment of AL amyloidosis. Bone Marrow Transplant 2001; 28: 637–642.
Madan S, Kumar SK, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al. High-dose melphalan and peripheral blood stem cell transplantation for light-chain amyloidosis with cardiac involvement. Blood 2012; 119: 1117–1122.
Girnius S, Seldin DC, Meier-Ewert HK, Sloan JM, Quillen K, Ruberg FL et al. Safety and efficacy of high-dose melphalan and auto-SCT in patients with AL amyloidosis and cardiac involvement. Bone Marrow Transplant 2013; 49: 434–439.
Mhaskar R, Kumar A, Behera M, Kharfan-Dabaja MA, Djulbegovic B . Role of high-dose chemotherapy and autologous hematopoietic cell transplantation in primary systemic amyloidosis: a systematic review. Biol Blood Marrow Transplant 2009; 15: 893–902.
Sanchorawala V, Hoering A, Seldin DC, Finn KT, Fennessey SA, Sexton R et al. Modified high-dose melphalan and autologous SCT for AL amyloidosis or high-risk myeloma: analysis of SWOG trial S0115. Bone Marrow Transplant 2013; 48: 1537–1542.
Rosenzweig M, Giralt S, Landau H . Light-chain amyloidosis: SCT, novel agents and beyond. Bone Marrow Transplant 2013; 48: 1022–1027.
Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 2005; 79: 319–328.
Rajkumar SV . Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol 2011; 86: 57–65.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53 457–481.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol 2012; 30: 989–995.
Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013; 121: 3420–3427.
Ogura M, Sekine R, Nishiyama S, Abe Y, Iizuka H, Kusaka S et al. [Clinical analysis of autologous stem cell transplantation for nine cases of cardiac amyloidosis]. Rinsho Ketsueki 2012; 53: 710–715.
Gertz M, Lacy M, Dispenzieri A, Hayman S, Kumar S, Buadi F et al. Troponin T level as an exclusion criterion for stem cell transplantation in light-chain amyloidosis. Leuk Lymphoma 2008; 49: 36–41.
Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Dingli D, Leung N et al. Refinement in patient selection to reduce treatment-related mortality from SCT in amyloidosis. Bone Marrow Transplant 2012; 48: 557–561.
Bleeker JS, Gertz MA, Pellikka PA, Larson DR, Buadi F, Dingli D et al. Evaluation of pretransplant factors predicting cardiac dysfunction following high-dose melphalan conditioning and autologous peripheral blood stem cell transplantation. Eur J Haematol 2012; 89: 228–235.
Mikhael JR, Schuster SR, Jimenez-Zepeda VH, Bello N, Spong J, Reeder CB et al. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood 2012; 119: 4391–4394.
Seldin DC, Anderson JJ, Skinner M, Malek K, Wright DG, Quillen K et al. Successful treatment of AL amyloidosis with high-dose melphalan and autologous stem cell transplantation in patients over age 65. Blood 2006; 108: 3945–3947.
Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman SR, Buadi FK et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc 2011; 86: 12–18.
Gertz MA, Lacy MQ, Dispenzieri A, Kumar S, Hayman SR, Buadi F et al. Trend toward improved day 100 and two-year survival following stem cell transplantation for AL: a comparison before and after 2006. Amyloid 2011; 18 (Suppl 1): 137–138.
Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Buadi FK, Dingli D et al. Trends in day 100 and 2-year survival after auto-SCT for AL amyloidosis: outcomes before and after 2006. Bone Marrow Transplant 2011; 46: 970–975.
Chaulagain CP, Comenzo RL . New insights and modern treatment of Al amyloidosis. Curr Hematol Malig Rep 2013; 8: 291–298.
Palladini G, Russo P, Milani P, Foli A, Lavatelli F, Nuvolone M et al. A phase II trial of cyclophosphamide, lenalidomide and dexamethasone in previously treated patients with AL amyloidosis. Haematologica 2013; 98: 433–436.
Acknowledgements
We thank Joshua Howell, PharmD, Mubeen A Khan and Hilal Gunaydin, MD for data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Parmar, S., Kongtim, P., Champlin, R. et al. Auto-SCT improves survival in systemic light chain amyloidosis: a retrospective analysis with 14-year follow-up. Bone Marrow Transplant 49, 1036–1041 (2014). https://doi.org/10.1038/bmt.2014.115
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.115
This article is cited by
-
A comprehensive overview of AL amyloidosis disease characteristics accumulated over two decades at a single referral center in Korea
International Journal of Hematology (2022)
-
Superior efficacy but higher cost of plerixafor and abbreviated-course G-CSF for mobilizing hematopoietic progenitor cells (HPC) in AL amyloidosis
Bone Marrow Transplantation (2015)
-
Treatment of myeloma patients with renal impairment
memo - Magazine of European Medical Oncology (2015)