Abstract
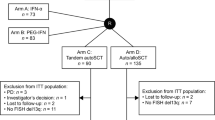
There is no standard therapy for multiple myeloma relapsing after an autotransplant. We compared the outcomes of a second autotransplant (N=137) with those of an allotransplant (N=152) after non-myeloablative or reduced-intensity conditioning (NST/RIC) in 289 subjects reported to the CIBMTR from 1995 to 2008. NST/RIC recipients were younger (median age 53 vs 56 years; P<0.001) and had a shorter time to progression after their first autotransplant. Non-relapse mortality at 1-year post transplant was higher in the NST/RIC cohort, 13% (95% confidence interval (CI), 8–19) vs 2% (95% CI, 1–5, P⩽0.001). Three-year PFS and OS for the NST/RIC cohort were 6% (95% CI, 3–10%) and 20% (95% CI, 14–27%). Similar outcomes for the autotransplant cohort were 12% (95% CI, 7–19%, P=0.038) and 46% (95% CI, 37–55%, P=0.001). In multivariate analyses, risk of death was higher in NST/RIC recipients (hazard ratio (HR) 2.38 (95% CI, 1.79–3.16), P<0.001), those with Karnofsky performance score<90 (HR 1.96 (95% CI, 1.47–2.62), P<0.001) and transplant before 2004 (HR 1.77 (95% CI, 1.34–2.35) P⩽0.001). In conclusion, NST/RIC was associated with higher TRM and lower survival than an autotransplant. As disease status was not available for most allotransplant recipients, it is not possible to determine which type of transplant is superior after autotransplant failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rajkumar SV . Treatment of multiple myeloma. Nat Rev Clin Oncol 2011; 8: 479–491.
Jacubowiak J . Management strategies for relapsed/refractory multiple myeloma: current clinical perspectives. Semin Hematol 2012; 49 (Suppl 1): S16–S32.
Kumar SK, Lee JH, LaHuerta JJ, Morgan G, Richardson PG, Crowley J et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 2012; 26: 149–157.
Bensinger W, Rotta M, Storer B, Chauncey T, Holmberg L, Becker P et al. Allo-SCT for multiple myeloma: a review of outcomes at a single transplant center. Bone Marrow Transplant 2012; 47: 1312–1317.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant 2008; 14: 748–758.
Klein J, Moeschberger M . Survival Analysis: Techniques of Censored and Truncated Data 2nd edn. Springer-Verlag: New York, NY, USA, 2003).
Kaplan E . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Cox DR . Regression models and life tables. J R Stat Soc 1972; 34: 187–220.
Burzynski JA, Toro JJ, Patel RC, Lee S, Greene RE, Ochoa-Bayona JL et al. Toxicity of a second autologous peripheral blood transplant in patients with relapsed or recurrent multiple myeloma. Leuk Lymphoma 2009; 50: 1442–1447.
Olin RL, Vogl DT, Porter DL, Luger SM, Schuster SJ, Tsai DE et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant 2009; 43: 417–422.
Fenk R, Liese V, Neubauer F, Bruns I, Kondakci M, Balleisen S et al. Predictive factors for successful salvage high-dose therapy in patients with multiple myeloma relapsing after autologous blood stem cell transplantation. Leuk Lymphoma 2011; 52: 1455–1462.
Jimenez-Zepeda VH, Mikhael J, Winter A, Franke N, Masih-Khan E, Trudel S et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: impact on progression-free and overall survival. Biol Blood Marrow Transplant 2012; 18: 773–779.
Shah N, Ahmed F, Bashir Q, Qureshi S, Dinh Y, Rondon G et al. Durable remissions with salvage second autotransplants in patients with multiple myeloma. Cancer 2012; 118: 3549–3555.
Chow AW, Lee CH, Hiwase DK, To LB, Horvath N . Relapsed multiple myeloma: who benefits from salvage autografts? Intern Med J 2013; 43: 156–161.
Michaelis LC, Saad A, Zhong X, Le-Rademacher J, Freytes CO, Marks DI et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant. 2013; 19: 760–766.
Badros A, Barlogie B, Morris C, Desikan R, Martin SR, Munshi N et al. High response rate in refractory and poor-risk multiple myeloma after allotransplantation using a nonmyeloablative conditioning regimen and donor lymphocyte infusions. Blood 2001; 97: 2574–2579.
Kroger N, Sayer HG, Schwerdtfeger R, Kiehl M, Nagler A, Renges H et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood 2002; 100: 3919–3924.
Einsele H, Schafer HJ, Hebart H, Bader P, Meisner C, Plasswilm L et al. Follow-up of patients with progressive multiple myeloma undergoing allografts after reduced-intensity conditioning. Br J Haematol 2003; 121: 411–418.
Gerull S, Goerner M, Benner A, Hegenbart U, Klein U, Schaefer H et al. Long-term outcome of nonmyeloablative allogeneic transplantation in patients with high-risk multiple myeloma. Bone Marrow Transplant 2005; 36: 963–969.
Pérez-Simón JA, Sureda A, Fernández-Aviles F, Sampol A, Cabrera JR, Caballero D et al. Reduced-intensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia 2006; 20: 542–545.
Qazilbach MH, Saliba R, De Lima M, Hosing C, Couriel D, Aleman A et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer 2006; 106: 1084–1089.
Georges GE, Maris MB, Maloney DG, Sandmaier BM, Sorror ML, Shizuru JA et al. Nonmyeloablative unrelated donor hematopoietic cell transplantation to treat patients with poor-risk, relapsed, or refractory multiple myeloma. Biol Blood Marrow Transplant 2007; 13: 423–432.
Majolino I, Davoli M, Carnevalli E, Locasciulli A, Di Bartolomeo P, Scime R et al. Reduced intensity conditioning with thiotepa, fludarabine, and melphalan is effective in advanced multiple myeloma. Leuk Lymphoma 2007; 48: 759–766.
De Lavallade H, El-Cheikh J, Faucher C, Furst S, Stoppa AM, Coso D et al. Reduced-intensity conditioning allogeneic SCT as salvage treatment for relapsed multiple myeloma. Bone Marrow Transplant 2008; 41: 953–960.
Osman K, Elliot B, Mandeli J, Scigliano E, Malone A, Isola L et al. Non-myeloablative conditioning and allogeneic transplantation for multiple myeloma. Am J Hematol 2010; 85: 249–254.
Shimoni A, Hardan I, Ayuk F, Schilling G, Atanackovic D, Zeller W et al. Allogenic hematopoietic stem cell transplantation with reduced-intensity conditioning in patients with refractory and recurrent multiple myeloma. Cancer 2010; 116: 3621–3630.
Crawley C, Lallancete M, Szydlo R, Gilleece M, Peggs K, Mackinnon S et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors of the Leukemia Working Party of the EBMT. Blood 2005; 105: 4532–4539.
Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea III E et al. Tandem autologous versus single autologous transplantation followed by allogeneic hematopoietic cell transplantation for patients with multiple myeloma: results from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0102 trial. Lancet Oncol 2011; 12: 1195–1203.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia and Lymphoma Society; The Medical College of Wisconsin; Merck and Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi, US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense or any other agency of the US Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented, in part, at the American Society of Hematology 53rd Annual Scientific Meeting held in San Diego, CA, USA from December 10–13, 2011.
Rights and permissions
About this article
Cite this article
Freytes, C., Vesole, D., LeRademacher, J. et al. Second transplants for multiple myeloma relapsing after a previous autotransplant—reduced-intensity allogeneic vs autologous transplantation. Bone Marrow Transplant 49, 416–421 (2014). https://doi.org/10.1038/bmt.2013.187
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.187
Keywords
This article is cited by
-
Current Role of Allogeneic Stem Cell Transplantation in Multiple Myeloma
Oncology and Therapy (2022)
-
Autologous hematopoietic cell transplantation for relapsed multiple myeloma performed with cells procured after previous transplantation–study on behalf of CMWP of the EBMT
Bone Marrow Transplantation (2022)
-
A clinical perspective on plasma cell leukemia; current status and future directions
Blood Cancer Journal (2021)
-
Allogeneic Stem Cell Transplantation in Patients with High-Risk Multiple Myeloma: Utopia or Continuous Challenge in Aiming for Cure?
Current Treatment Options in Oncology (2021)
-
Long-term survival and polyclonal immunoglobulin reconstitution after allogeneic stem cell transplantation in multiple myeloma
Annals of Hematology (2020)