Abstract
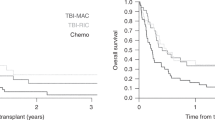
The aim of this study was to identify risk factors associated with PFS in patients with Ewing sarcoma undergoing ASCT; 116 patients underwent ASCT in 1989–2000 and reported to the Center for International Blood and Marrow Transplant Research. Eighty patients (69%) received ASCT as first-line therapy and 36 (31%), for recurrent disease. Risk factors affecting ASCT were analyzed with use of the Cox regression method. Metastatic disease at diagnosis, recurrence prior to ASCT and performance score <90 were associated with higher rates of disease recurrence/progression. Five-year probabilities of PFS in patients with localized and metastatic disease at diagnosis who received ASCT as first-line therapy were 49% (95% CI 30–69) and 34% (95% CI 22–47) respectively. The 5-year probability of PFS in patients with localized disease at diagnosis, and received ASCT after recurrence was 14% (95% CI 3–30). PFS rates after ASCT are comparable to published rates in patients with similar disease characteristics treated with conventional chemotherapy, surgery and irradiation suggesting a limited role for ASCT in these patients. Therefore, ASCT if considered should be for high-risk patients in the setting of carefully controlled clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ginsberg JP, Woo SY, Johnson ME, Hicks MJ, Horowitz ME . Ewing's sarcoma family of tumors: Ewing's sarcoma of bone soft tissue and the peripheral primitive neuroectodermal tumors. In: Pizzo and Poplack. Lippincott Williams and Wilkins: Philadelphia, PA, 2002, pp 973–1016.
Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003; 348: 694–701.
Cangir A, Vietti TJ, Gehan EA, Burgert Jr EO, Thomas P, Tefft M et al. Ewing's sarcoma metastatic at diagnosis. Results and comparisons of two intergroup Ewing's sarcoma studies. Cancer 1990; 66: 887–893.
Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ et al. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide—A Children's Cancer Group and Pediatric Oncology Group Study. J Clin Oncol 2004; 22: 2873–2876.
Paulussen M, Ahrens S, Craft AW, Dunst J, Frohlich B, Jabar S et al. Ewing's tumors with primary lung metastases: survival analysis of 114 (European Intergroup) Cooperative Ewing's Sarcoma Studies patients. J Clin Oncol 1998; 16: 3044–3052.
Shankar AG, Ashley S, Craft AW, Pinkerton CR . Outcome after relapse in an unselected cohort of children and adolescents with Ewing sarcoma. Med Pediatr Oncol 2003; 40: 141–147.
Rodriguez-Galindo C, Billups CA, Kun LE, Rao BN, Pratt CB, Merchant TE et al. Survival after recurrence of Ewing tumors: the St Jude Children's Research Hospital experience, 1979–1999. Cancer 2002; 94: 561–569.
Ahrens S, Hoffmann C, Jabar S, Braun-Munzinger G, Paulussen M, Dunst J et al. Evaluation of prognostic factors in a tumor volume-adapted treatment strategy for localized Ewing sarcoma of bone: the CESS 86 experience. Cooperative Ewing Sarcoma Study. Med Pediatr Oncol 1999; 32: 186–195.
Wunder JS, Paulian G, Huvos AG, Heller G, Meyers PA, Healey JH et al. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am 1998; 80: 1020–1033.
de Alava E, Kawai A, Healey JH, Fligman I, Meyers PA, Huvos AG et al. EWS-FLI1 fusion transcript structure is an independent determinant of prognosis in Ewing's sarcoma. J Clin Oncol 1998; 16: 1248–1255.
Zoubek A, Dockhorn-Dworniczak B, Delattre O, Christiansen H, Niggli F, Gatterer-Menz I et al. Does expression of different EWS chimeric transcripts define clinically distinct risk groups of Ewing tumor patients? J Clin Oncol 1996; 14: 1245–1251.
Burdach S, Jurgens H, Peters C, Nurnberger W, Mauz-Korholz C, Korholz D et al. Myeloablative radiochemotherapy and hematopoietic stem-cell rescue in poor-prognosis Ewing's sarcoma. J Clin Oncol 1993; 11: 1482–1488.
Fraser CJ, Weigel BJ, Perentesis JP, Dusenbery KE, DeFor TE, Baker KS et al. Autologous stem cell transplantation for high-risk Ewing's sarcoma and other pediatric solid tumors. Bone Marrow Transplant 2006; 37: 175–181.
Drabko K, Zawitkowska-Klaczynska J, Wojcik B, Choma M, Zaucha-Prazmo A, Kowalczyk J et al. Mega chemotherapy followed by autologous stem cell transplantation in children with Ewing's sarcoma. Pediatr Transplant 2005; 9: 618–621.
Atra A, Whelan JS, Calvagna V, Shankar AG, Ashley S, Shepherd V et al. High-dose busulphan/melphalan with autologous stem cell rescue in Ewing's sarcoma. Bone Marrow Transplant 1997; 20: 843–846.
Ladenstein R, Lasset C, Pinkerton R, Zucker JM, Peters C, Burdach S et al. Impact of mega therapy in children with high-risk Ewing's tumours in complete remission: a report from the EBMT Solid Tumour Registry. Bone Marrow Transplant 1995; 15: 697–705.
Klein JP, Rizzo JD, Zhang MJ, Keiding N . Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: regression modeling. Bone Marrow Transplant 2001; 28: 1001–1011.
Kaplan E . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life tables. J R Stat Soc 1972; 34: 187.
Therneau T, Grambasch P . Modeling Survival Data: Extending the Cox Model. Springer Verlag: New York, NY, 2000.
Andersen PK, Klein JP, Zhang MJ . Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med 1999; 18: 1489–1500.
Horowitz ME, Kinsella TJ, Wexler LH, Belasco J, Triche T, Tsokos M et al. Total-body irradiation and autologous bone marrow transplant in the treatment of high-risk Ewing's sarcoma and rhabdomyosarcoma. J Clin Oncol 1993; 11: 1911–1918.
Meyers PA, Krailo MD, Ladanyi M, Chan KW, Sailer SL, Dickman PS et al. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing's sarcoma does not improve prognosis. J Clin Oncol 2001; 19: 2812–2820.
Acknowledgements
This work was supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Services Research Administration (DHHS); and grants from AABB; Abbott Laboratories; Aetna; American International Group Inc.; Amgen Inc.; Anonymous donation to the Medical College of Wisconsin; AnorMED Inc.; Astellas Pharma US Inc.; Baxter International Inc.; Berlex Laboratories Inc.; Biogen IDEC Inc.; BioOne Corporation; Blood Center of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Bristol-Myers Squibb Company; Cangene Corporation; Celgene Corporation; CellGenix GmbH; Cerus Corporation; Cylex Inc.; CytoTherm; DOR BioPharma Inc.; Dynal Biotech, an Invitrogen Company; EKR Therapeutics; Enzon Pharmaceuticals Inc.; Gambro BCT Inc.; Gamida Cell Ltd.; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline Inc.; Histogenetics Inc.; HKS Medical Information Systems; Hospira Inc.; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec Inc.; MultiPlan Inc.; National Marrow Donor Program; Nature Publishing Group; Oncology Nursing Society; Osiris Therapeutics Inc.; Pall Life Sciences; PDL BioPharma Inc.; Pfizer Inc.; Pharmion Corporation; Roche Laboratories; Sanofi-aventis; Schering Plough Corporation; StemCyte Inc.; StemSoft Software Inc.; SuperGen Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS Inc.; University of Colorado Cord Blood Bank; ViaCell Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma Inc.; Wellpoint Inc.; and Zelos Therapeutics Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gardner, S., Carreras, J., Boudreau, C. et al. Myeloablative therapy with autologous stem cell rescue for patients with Ewing sarcoma. Bone Marrow Transplant 41, 867–872 (2008). https://doi.org/10.1038/bmt.2008.2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.2
Keywords
This article is cited by
-
Autologous stem cell transplantation in adults with metastatic sarcoma of the Ewing family: a single centre experience
Wiener klinische Wochenschrift (2013)
-
Consolidation of first-line therapy with busulphan and melphalan, and autologous stem cell rescue in children with Ewing’s sarcoma
Bone Marrow Transplantation (2012)
-
Myeloablative chemotherapy with autologous stem cell rescue for Ewing sarcoma
Bone Marrow Transplantation (2008)
-
Response to Dr Gilman
Bone Marrow Transplantation (2008)