In long-term care facilities, infections are a common cause of morbidity and mortality(Reference Richards1). Infections in the elderly are more frequent and severe than in the general population(Reference Gavazzi and Krause2). Individuals in nursing homes have a higher risk of acquiring infectious diseases compared with community-dwelling individuals, and pneumonia is the leading cause of morbidity and mortality in this group(Reference Furman, Rayner and Tobin3).
Among geriatric subjects, a low BMI and unintentional weight loss are a great mortality threat(Reference Locher, Roth and Ritchie4). A high BMI and obesity, on the other hand, are an emerging phenomenon not only in the general community, but also in geriatric populations(Reference Eiben, Dey and Rothenberg5) and in nursing homes(Reference Lapane and Resnik6). A high BMI has been shown to be associated with a lower mortality risk in older subjects: in the Longitudinal Study of Aging among 7527 participants aged 70 years and older, individuals with a BMI of 28·5 kg/m2 and more had a significantly lower risk for death compared with those with a BMI between 18·5 and 28·4 kg/m2(Reference Grabowski and Ellis7). Among geriatric in-patients with a mean age of 81·5 years, the age-adjusted mortality rate decreased from 24 to 9·6 per 100 patient-years from the highest BMI quartile (BMI ≥ 28 kg/m2) to the lowest (BMI < 22 kg/m2)(Reference Weiss, Beloosesky and Boaz8). However, in subjects aged 65 years or older, a BMI>27 kg/m2 has been shown to be associated with an increasing mortality risk(Reference Heiat, Vaccarino and Krumholz9). Obesity in older subjects is also associated with increased functional limitations and poor quality of life(Reference Davison, Ford and Cogswell10, Reference Jensen11), and obese older individuals are admitted more frequently to nursing homes compared with those who are not obese(Reference Zizza, Herring and Stevens12).
Data about the influence of BMI on the incidence of infections are scarce. In the present analysis we wanted to determine the frequency of infections among institutionalised geriatric patients. The aim of the present study was to clarify the impact of the baseline BMI on the development of infectious diseases in this particular population. It was not the purpose to investigate underlying mechanisms of infectious diseases.
Methods
Subjects and data
The data in the present study are based on a retrospective analysis of medical records of patients who were treated at the geriatric hospital ‘Haus der Barmherzigkeit’ in Vienna, Austria. This hospital is specialised in the medical and nursing support of multi-morbid geriatric patients who require a high level of care. Medical records were documented by the ward physician with the use of electronic patients' charts (‘Geripas’)(13) and included variables such as age and sex, diagnoses of chronic diseases, and anthropometric parameters such as body height and body weight which were measured routinely by the ward staff. Body weight was measured with a regularly calibrated person weight scale or a chair scale. Body height was assessed in standing or lying position with a measuring tape. Furthermore the development of acute diseases that warrant medical interventions, such as infectious diseases, were also documented by the ward physician. As a general rule, infections of the lower respiratory tract were diagnosed clinically and verified by X-ray, urinary tract infections (UTI) were diagnosed by nitrite positive stick in the case of clinical suspicion, usually followed by urine culture, and diarrhoea was diagnosed clinically, optionally followed by stool culture.
For this analysis, only subjects aged 75 years and above with a complete set of data regarding body weight, body height, age and period under observation were included. Incidence rates were computed as number of events per person-year. We examined in particular the association between BMI and the occurrence of several types of infections (infections of the lower respiratory tract, UTI, diarrhoea, and other infections). For this purpose four BMI groups were set up ( < 20, 20–23·9, 24–27·9 and ≥ 28 kg/m2). We did not use WHO(14) or National Institutes of Health(15) cut-off points, in order to allow a sufficient sample size in each BMI category.
Statistics
Descriptive statistics were applied; mean values are presented with standard deviations. The main outcome measures were incidence risk ratios that were obtained by a multiplicative Poisson regression model, which was fitted as a log-linear regression (a log link and a Poisson error distribution), with an offset equal to the natural logarithm of exposure time. These incidence rates are presented with 95 % CI.
To estimate and visualise the precise pattern of the association between BMI and incidence of infection, we employed a generalised additive model with a log link and a Poisson error distribution. In the model the dependent variable y was a function of the predictor BMI, but their association was not necessarily linear:
The term E(y) divided by person time (PT) represents the estimated incidence rate and c is the constant in the model. Generalised additive models allow the fitting of any predictor with a non-parametric smoothing function; hence, no a priori assumption regarding the pattern of the predictor's effect is required. The following covariables for applying the multivariate Poisson models were used: sex (female), age ( ≥ the median of 88 years) and the presence of nine different chronic medical conditions. All calculations were carried out using the R Language and Environment for Statistical Computing and Graphics, version 2.7.2 (http://www.r-project.org/).
Results
The records of 784 subjects were available. Excluded were 124 patients for failure to meet the inclusion criterion of at least 75 years of age. Also excluded were forty-one subjects because of missing data; thus a total of 619 subjects were included for further analyses. These subjects were followed for a mean period of 368 (sd 209) d (median 398 d; range 2–595 d), resulting in a total exposure time of 624·6 person-years, 94·2 male and 530·4 female person-years. There were 188·1 person-years of subjects with a BMI of under 20 kg/m2, 231·1 person-years of subjects with a BMI between 20 and 23·9 kg/m2, 134·8 person-years with a BMI between 24 and 27·9 kg/m2, and 70·6 person-years of subjects with a BMI equal to or greater than 28 kg/m2.
The baseline characteristics of study participants such as age, BMI and prevalence of chronic diseases for men, for women and in total are presented in Table 1. During the period of observation a total of 500 infectious diseases were recorded, resulting in an incidence rate of 0·80 per person-year. The incidence rates were 0·97 per person-year for men and 0·77 per person-year for women. Older subjects ( ≥ 88 years) had a slightly higher incidence rate of infections (0·82 per person-year) compared with younger subjects (0·77 per person-year). Of the 500 infections recorded, 120 were infections of the lower respiratory tract, 185 were UTI, seventy-three cases of diarrhoea and 122 other infections. The corresponding incidence rates were 0·19, 0·30, 0·12 and 0·20 per person-year, respectively. The incidence rates for the men were 0·25, 0·47, 0·07 and 0·17 and for the women 0·18, 0·27, 0·12 and 0·20 per person-year, respectively.
Table 1 Baseline characteristics of 619 institutionalised geriatric patients aged 75 years and older
(Number of subjects and percentages or mean values and standard deviations)

The association between BMI and incidence of infections is depicted in Fig. 1. Patients with a BMI between 24 and 27·9 kg/m2 had the lowest incidence rate for infections and were defined as the reference group. Subjects with a lower BMI had a higher incidence rate of infections, with raw incidence risk ratios based on univariate Poisson models of 1·67 (95 % CI 1·25, 2·21) for those with a BMI of < 20 kg/m2 and 1·90 (95 % CI 1·45, 2·49) for those with a BMI of 20–23·9 kg/m2. However, also those patients with a BMI of 28 kg/m2 and above had a higher incidence rate of infectious diseases with a raw incidence risk ratio of 1·49 (95 % CI 1·04, 2·14).
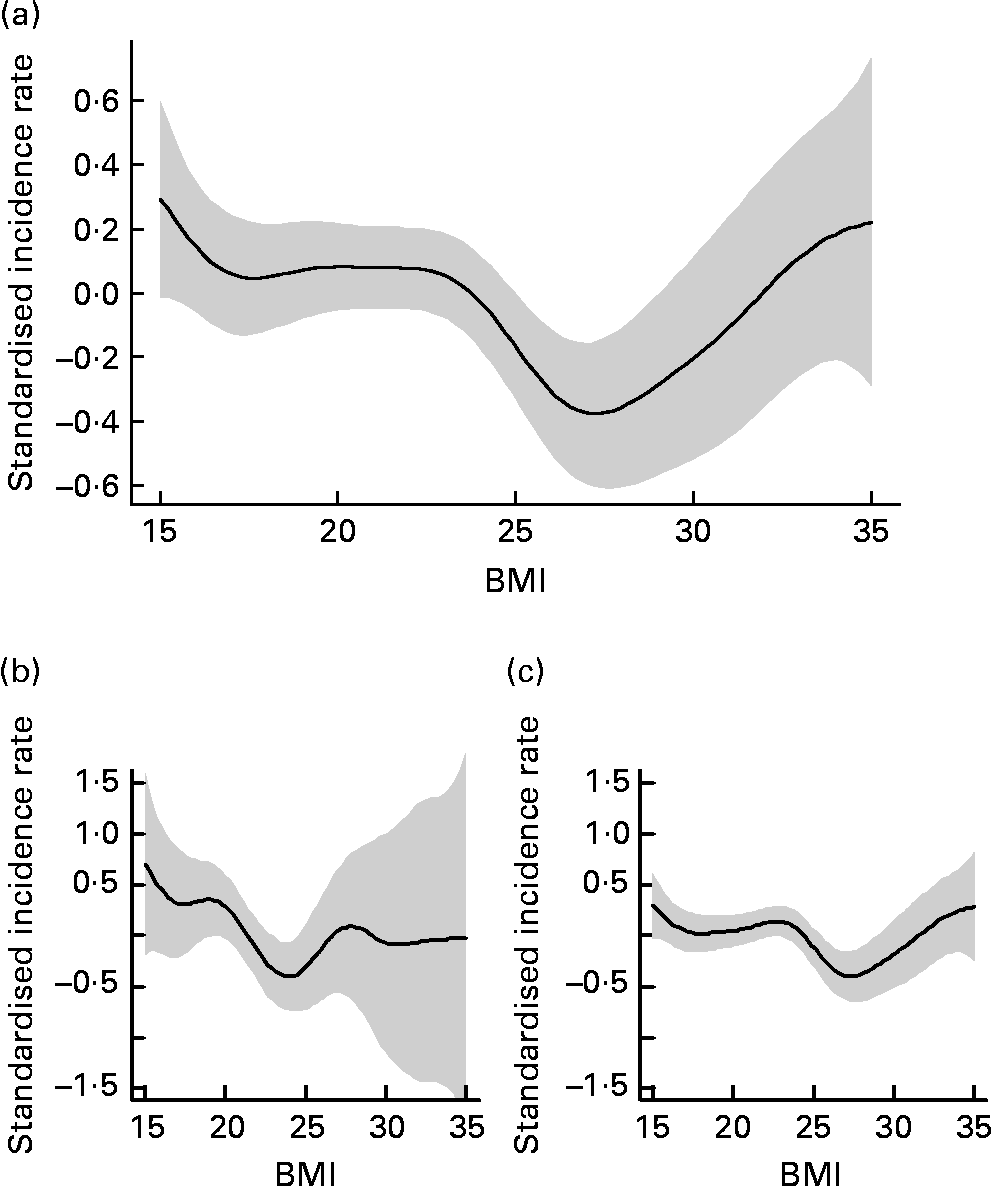
Fig. 1 Estimated association between BMI and incidence for infections (![]() ), with 2 se confidence band (
), with 2 se confidence band (![]() ) in institutionalised geriatric patients aged 75 years and older. (a) All subjects; (b) men; (c) women.
) in institutionalised geriatric patients aged 75 years and older. (a) All subjects; (b) men; (c) women.
Controlling for sex, age and chronic diseases had little impact on the above described picture, as presented in Table 2, based on multivariate Poisson models. Also after adjusting, the data showed a significantly higher incidence risk ratio for infections in the BMI groups of < 20, 20–23·9 and ≥ 28 kg/m2. Female sex was a protective factor for UTI. A higher age was associated with a higher chance of diarrhoea. Dementia was associated with a higher risk of ‘other infections’. Parkinson disease was significantly associated with all infections and with infections of the lower respiratory tract, and chronic obstructive pulmonary disease was also associated with infections of the lower respiratory tract.
Table 2 Risk, based on multivariate Poisson models, for total infections, infections of the lower respiratory tract, urinary tract infections, diarrhoea and other infections in institutionalised geriatric patients aged 75 years and older, adjusted for sex, age and chronic diseases
(Incidence risk ratios (IRR) and 95 % confidence intervals)

Discussion
Depending on the BMI, the incidence of infections in institutionalised geriatric patients shows a J-shaped curve, with a high incidence rate in underweight and normal-weight subjects, but also an increasing incidence with increasing BMI in obese subjects. The nadir of this curve was found at a BMI of about 27–28 kg/m2. This curve resembles the J-shaped curve of mortality depending on the BMI in the general population. Most analyses show that in the general population both a low and a very high BMI are associated with increased overall as well as cause-specific mortality(Reference Calle, Thun and Petrelli16–Reference Flegal, Graubard and Williamson18). The nadir of this J-shaped curve was found at a BMI of 23·5 to 24·9 kg/m2 in men and 22·0 to 23·4 kg/m2 in women(Reference Calle, Thun and Petrelli16). For older individuals, also a U- or J-shaped mortality curve depending on the BMI has been described with the difference that this curve is shifted to the right. In the elderly population (aged 50–80 years), the nadir of the mortality curve was found at a BMI of 28·2 kg/m2 for men and 27·1 kg/m2 for women(Reference Zajacova19). In the population aged 65 years or older, overweight was not found to be associated with a higher mortality risk, whereas mortality increased with a BMI of 31 kg/m2 and higher(Reference Heiat, Vaccarino and Krumholz9).
In our analyses we found an incidence rate of infection of 0·80 per person-year, the most frequent infections being UTI. In a French care unit housing residents with a mean age of 82 years, the incidence rate of infections was more than three times higher (7·6 per 1000 bed-days); most of them, here too, were UTI. In this analysis, subjects with infections had an altered nutritional status(Reference Paillaud, Herbaud and Caillet20).
After controlling for age, sex and chronic diseases, the same pattern for the incidence rate of infections could be observed as in the analyses of the crude data. It is interesting to see that age and most chronic diseases, for example, the diagnosis of diabetes mellitus, did not influence the incidence of infections in our population. This fact is likely to be an indicator for the good glycaemic control in patients with diabetes in our cohort. There was a sex difference in the incidence rate of UTI: men were unexpectedly more frequently affected by UTI than women. Usually the prevalence ratio between women and men in older individuals is 2:1(Reference Yoshikawa, Nicolle and Norman21). Common reasons for UTI in the elderly are mechanical changes in the bladder, urothelial changes, lack of oestrogen in postmenopausal women, prostatic hyperplasia in men(Reference Gavazzi and Krause2), but also urinary catheters(Reference Moro, Mongardi and Marchi22). The higher chance for UTI in our sample could be due to the fact that more men than women were supplied with urinary catheters, or to a high prevalence of prostate hyperplasia in men; data to confirm these assumptions are, however, missing. Dementia was also found to be associated with a higher rate of infections. This also is in line with the findings of previous studies(Reference Challa, Sharkey and Chen23, Reference Kagansky, Berner and Koren-Morag24).
Among institutionalised geriatric patients, nutrition-related problems are very common. The extremes of the BMI range can be regarded as indicators for nutritional problems in that undernutrition can lead to sarcopenia and a low BMI, while overnutrition can lead to obesity and a high BMI. Malnutrition, a chronic state in which a combination of varying degrees of over- and undernutrition and inflammatory activity change body composition(Reference Soeters, Reijven and van Bokhorst-de van der Schueren25, Reference Soeters and Schols26), is associated with increased mortality in very old hospitalised patients(Reference Kagansky, Berner and Koren-Morag24). Malnutrition is also a risk factor for infections in older individuals(Reference Gavazzi and Krause2), and a poor nutritional status is an indicator of adverse prognosis for pneumonia in the elderly population(Reference Janssens27).
Undernutrition is defined as a state of energy, protein or other specific nutrient deficiency which produces a measurable change in body function and is associated with worse outcome from illness(Reference Allison28). In undernourished subjects, the missing micronutrients and proteins are a major cause of a decreased immune function. Hence undernutrition makes especially geriatric subjects prone to infections. Undernutrition has been shown to be a risk factor for nosocomial pneumonia in a geriatric hospital(Reference Rothan-Tondeur, Meaume and Girard29). On the other hand, infections can also cause a decrease in BMI in geriatric subjects with little nutritional reserve, which in turn, as a vicious cycle, enhances the probability of a more severe course of infection and of the acquisition of additional infections(Reference Gavazzi and Krause2).
However, also a high BMI or obesity has a clear effect on the immune response through immune mediators that lead to susceptibility to infections. Obesity has been shown to increase the risk especially for nosocomial infections and postoperative infections(Reference Falagas and Kompoti30).
Underweight and obesity are the respective extremes on the BMI scale; however, some pathophysiological mechanisms occur in both cases. Underweight(Reference Challa, Sharkey and Chen23, Reference Woods, LaCroix and Gray31) and obesity(Reference Woods, LaCroix and Gray31–Reference Villareal, Banks and Siener33) are both associated with frailty in geriatric subjects, and frailty is associated with infection and with inflammation(Reference Schmaltz, Fried and Xue34, Reference Walston, Hadley and Ferrucci35). Frailty is defined as a state of increased vulnerability to stressors(Reference Walston, Hadley and Ferrucci35) and therefore frailty is also associated with a loss of resilience against infectious agents(Reference Varadhan, Seplaki and Xue36). Sarcopenia and sarcopenic obesity are common aspects of frailty in older subjects(Reference Zamboni, Mazzali and Fantin37), and sarcopenia doubles the risk of nosocomial infections(Reference Cosquéric, Sebag and Ducolombier38). So, frailty and chronic inflammation can be the common factors associated with the higher proportion of infectious diseases in underweight as well as obese patients. Functional impairment in nursing home residents can lead to a higher risk of infections(Reference Moro, Mongardi and Marchi22). On the other hand, infectious diseases can cause functional impairment(Reference Büla, Ghilardi and Wietlisbach39) and frailty in older individuals.
A limitation of the present study is its retrospective design. The change of BMI during the observation period of up to 20 months could not be taken into account. Since weight loss is included in the operational definition of the concept of frailty, the influence of a change of body weight over time on the risk of infections should be of particular interest in future research. The non-standardised measurement of body weight and body height can lead to possible inaccuracies in the calculation of BMI; however, the weight is likely to be correct to within a few kg, and bias from the non-standardised measurements is limited. The influence of nutrition treatment on the development of infections could not be observed, since there were no relevant data at the time of analysis. The use of BMI for classifying the nutritional status of elderly subjects does not take into account influences on body height such as osteoporosis, assessment of the compartments, fat or fat-free mass, that contribute to a high or a low body weight and as a consequence to BMI. Additionally, BMI does not take into account the role of fat distribution. The amount of observed person-time differs in the four BMI strata. This neither affects the incidence rates nor the incidence risk ratios. However, the number of obese subjects was clearly lower compared with subjects in other BMI strata, which results in large CI in this BMI stratum. This was especially marked in men, where the total number of subjects was already low; hence sex-specific conclusions based on our data are limited. Further research will be necessary to assess the influence that other factors related to nutritional status, such as body composition, have on the incidence of infections. The role of frailty and chronic inflammation in connection with BMI, nutritional status and the incidence of infections should be clarified further. Furthermore, the effects of intervention in order to optimise the nutritional status or to achieve a BMI of 24–27·9 kg/m2 – identified as the BMI category with the lowest risk for infections in geriatric subjects – require further research.
Despite potential limitations of the present study, the results show a clear association between BMI and the risk of infections in institutionalised geriatric patients. The lowest risk in this population occurs in the ‘overweight’ (according to the WHO and National Institutes of Health categories) subjects. A lower BMI and obesity both increase the risk of infections.
Acknowledgements
We would like to thank both the medical and nursing staff of the ‘Haus der Barmherzigkeit’ for making the present study possible. There were no sources of funding for the present study.
All authors made substantial contributions to the intellectual content of the manuscript and approved the final version. T. E. D. developed the study concept, developed the hypotheses, interpreted the results and drafted the manuscript. F. S. performed statistical analyses and interpreted the data. A. K. was involved in the development of electronic patients' charts, and contributed to the study concept and manuscript revision. W. F. and A. R. interpreted the data and were involved in manuscript revision. C. G. contributed to the study concept, data interpretation and manuscript revision.
The authors do not have any conflicts of interest.







