Abstract
Background
Plexiform neurofibromas (PN) are congenital tumors that affect up to 50% of individuals with neurofibromatosis type 1. Despite their benign nature, they can grow rapidly and cause severe morbidities. Selumetinib, an inhibitor of mitogen-activated protein kinase (MEK) 1 and 2, was reported to induce a clinical response in pediatric subjects with inoperable PN.
Objective
The aim of this paper is to describe a prospective case series of patients treated with selumetinib with emphasis on drug adverse events.
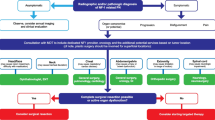
Patients and methods
All the subjects who received selumetinib at the Pediatric Department of Scientific Research Institute and Hospital “Burlo Garofolo”, from November 2017 to January 2020, were progressively included. We monitored the patients with a follow-up visit every 3 months. MRI or CT scans to monitor the growth of the tumor were performed after 3 months of treatment, and then every 6–9 months.
Results
Selumetinib was prescribed to nine children, with a total of 17 inoperable PN. The mean follow-up period was 12 months. During the follow-up, one patient experienced an ischemic stroke, unrelated to the treatment. Only minor adverse events were observed: six individuals developed gastrointestinal side effects, seven patients presented a mild form of acne, six had paronychia, four developed irritability, and two showed a mild increase in creatine kinase. None of the patients stopped the treatment. Tumor reduction > 20% was recorded in 16 out of 17 PN (94%). One PN remained stable. No tumor growth was recorded during the treatment.
Conclusions
In this case series, selumetinib appears to be effective and safe for the pediatric population.


Similar content being viewed by others
References
Ferner RE, Gutmann DH. Neurofibromatosis type 1 (NF1): diagnosis and management. Handb Clin Neurol. 2013;115:939–55.
Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis in German children at elementary school enrollment. Arch Dermatol. 2005;141:71–4.
Ruggieri M, Huson SM. The clinical and diagnostic implications mosaicism in the neurofibromatosis. Neurology. 2001;56:1433–43.
Friedman JM, Adam MP, Ardinger HH, Pagon RA, et al. Neurofibromatosis 1. Genereviews 1998 Oct 2 [Updated 2019 Jun 6].
Gutmann DH, Ferner RE, Listernick RH, Korf BR, Wolters PL, Johnson KJ. Neurofibromatosis type 1. Nat Rev Dis Prime. 2017;3:17004.
Dombi E, Solomon J, Gillespie AJ, et al. NF1 plexiform neurofibroma growth rate by volumetric MRI: relationship to age and body weight. Neurology. 2007;68:643–7.
Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7:75.
Ehara, Y., Koga, M., Imafuku, S., Yamamoto, O., Yoshida, Y. Distribution of diffuse plexiform neurofibroma on the body surface in patients with neurofibromatosis 1. J Dermatol. 2019. https://doi.org/10.1111/s1346813815194
Yoshida Y, Ehara Y, Koga M, Imafuku S, Yamamoto O. Epidemiological analysis of major complications requiring medical intervention in patients with neurofibromatosis 1. Acta Derm Venereol. 2018;98:753–6.
Friedman JM. Epidemiology of neurofibromatosis type 1. Am J Med Genet. 1999;89(1):1–6.
Nguyen R, Jett K, Harris GJ, et al. Benign whole body tumor volume is a risk factor for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. J Neurooncol. 2014;116(2):307–13.
Azizi AA, Slavc I, Theisen BE, et al. Monitoring of plexiform neurofibroma in children and adolescents with neurofibromatosis type 1 by [18 F]FDG-PET imaging. Is it of value in asymptomatic patients? Pediatr Blood Cancer. 2018;65(1).
Dunning-Davies BM, Parker APJ. Annual review of children with neurofibromatosis type 1. Arch Dis Child Educ Pract Ed. 2016;101(2):102–11.
Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:256–66.
Jakacki RI, Dombi E, Steinberg SM, et al. Phase II trial of pegylated interferon alfa-2b in young patients with neurofibromatosis type 1 and unresectable plexiform neurofibromas. Neuro Oncol. 2017;19(2):289–97.
Robertson KA, Nalepa G, Yang FC, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 2012;13:1218–24.
Dombi E, Baldwin A, Marcus LJ, et al. Activity of selumetinib in neurofibromatosis type 1 related plexiform neurofibromas. N Engl J Med. 2016;375(26):2550–60.
Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med. 2020 Apr 9;382(15):1430–42.
Takashi S, Fumihiko H, Hideo S, et al. Safety and tolerability of selumetinib as a monotherapy, or in combination with docetaxel as second-line therapy, in Japanese patients with advanced solid malignancies or non-small cell lung cancer. Jpn J Clin Oncol. 2018;48(1):31–42.
LoRusso PM, Infante JR, Kim KB, et al. A phase I dose-escalation study of selumetinib in combination with docetaxel or dacarbazine in patients with advanced solid tumors. BMC Cancer. 2017;17(1):173.
Fangusaro J, Onar-Thomas A, Young Poussaint T, et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type1-associated recurrent, refractory, or progressive low-grade glioma: a multicentre, phase 2 trial. Lancet Oncol. 2019;20(7):1011–22.
Espírito Santo V, Passos J, Nzwalo H, et al. Selumetinib for plexiform neurofibromas in neurofibromatosis type 1: a single-institution experience. J Neurooncol. 2020;147(2):459–63.
Passos J, Nzwalo H, Azevedo M, et al. Dramatic improvement of a massive plexiform neurofibroma after administration of selumetinib. Pediatr Neurol. 2020;105:69–70.
Papalia H, Audic F, Rivière GR, et al. Quick and sustained clinical response to MEK inhibitor I in a NF1 patient with neurofibromas. Ecancermedicalscience. 2018;12:862.
Vaassen P, Dürr N, Röhrig A, et al. Trametinib induces neurofibroma shrinkage and enables surgery. Neuropediatrics. 2019;50(5):300–3.
Perreault S, Larouche V, Tabori U, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway. BMC Cancer. 2019 Dec 27;19(1):1250.
Acknowledgements
The authors thank Martina Bradaschia MD, for the linguistic revision of the manuscript.
Author information
Authors and Affiliations
Contributions
FB collected and processed the data and wrote the manuscript. FB and AGG reviewed the literature. AGG and LCW co-wrote the manuscript. FMM and LB selected the clinical images. AM, MPPT, AM, IB, and EB reviewed the manuscript.
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflict of interest
Francesco Baldo, Antonio Giacomo Grasso, Luisa Cortellazzo Wiel, Alessandra Maestro, Marta Paulina Trojniak, Flora Maria Murru, Luca Basso, Andrea Magnolato, Irene Bruno, and Egidio Barbi declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethical approval
Approval was obtained from the ethics committee of our Institute.
Consent to participate
A written consent to participate was signed by all the subjects involved and/or their parents.
Consent for publication
A written consent for publication was signed by all the subjects involved and/or their parents.
Availability of data and material
All relevant data are included in the article and/or its supplementary information files. All other data supporting the study (such as radiological photos or images) are available from the corresponding author upon request.
Code availability
Data were collected and elaborated with Microsoft Office Excel.
Rights and permissions
About this article
Cite this article
Baldo, F., Grasso, A.G., Cortellazzo Wiel, L. et al. Selumetinib in the Treatment of Symptomatic Intractable Plexiform Neurofibromas in Neurofibromatosis Type 1: A Prospective Case Series with Emphasis on Side Effects. Pediatr Drugs 22, 417–423 (2020). https://doi.org/10.1007/s40272-020-00399-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-020-00399-y