Abstract
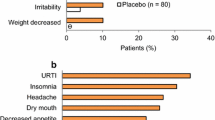
Lisdexamfetamine dimesylate (lisdexamfetamine; Elvanse®; Tyvense®), an orally-active dexamfetamine prodrug, is indicated in the EU for the treatment of attention-deficit hyperactivity disorder (ADHD) in children aged ≥ 6 years (including adolescents) when the response to previous methylphenidate (MPH) treatment is clinically inadequate. The original approval of the drug was based on the results of phase III trials in children and adolescents with ADHD who had an inadequate response to previous pharmacotherapy (e.g. MPH) or were treatment naïve. In these studies, short-term treatment with flexibly-dosed lisdexamfetamine demonstrated greater efficacy than atomoxetine, based on a prospective comparison, and osmotic-release oral system (OROS)-MPH, based on a post hoc comparison. Improvements in ADHD symptoms were accompanied by improvements in health-related quality of life and functioning that were maintained as long as treatment with lisdexamfetamine was continued in a long-term extension of one of these trials. In subsequent phase IV head-to-head studies in adolescents with ADHD and an inadequate response to previous pharmacotherapy, lisdexamfetamine demonstrated greater efficacy than OROS-MPH when both medications were force-titrated, but not when they were flexibly-titrated. Lisdexamfetamine was generally well tolerated, with an adverse event profile (e.g. decreased appetite, headache, weight reduction, insomnia and irritability) typical of that reported for other stimulants. Thus, lisdexamfetamine provides an alternative option for the treatment of children and/or adolescents with ADHD who have not responded adequately to previous ADHD pharmacotherapies.

Similar content being viewed by others
References
Adesman A. The diagnosis and management of attention-deficit/hyperactivity disorder in pediatric patients. Prim Care Companion J Clin Psychiatry. 2001;3(2):66–77.
Barkley RA. Attention-deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York: Guilford Press; 2006.
American Academy of Pediatrics. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007–22.
Danckaerts M, Sonuga-Barke EJS, Banaschewski T, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83–105.
Wu E, Hodgkins P, Ben-Hamadi R, et al. Cost effectiveness of pharmacotherapies for attention-deficit hyperactivity disorder. CNS Drugs. 2012;26(7):581–600.
Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8.
National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management. NICE guideline [NG87]. 2018. https://www.nice.org.uk. Accessed 5 June 2018.
Huss M, Chen W, Ludolph AG, et al. Guanfacine extended release: a new pharmacological treatment option in Europe. Clin Drug Investig. 2016;36:1–25.
Shier AC. Pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: clinical strategies. J Cent Nerv Syst Dis. 2013;5:1–17.
Luan R, Mu Z, Yue F, et al. Efficacy and tolerability of different interventions in children and adolescents with attention deficit hyperactivity disorder. Front Psychiatry. 2017;8:229. https://doi.org/10.3389/fpsyt.2017.00229.
Joseph A, Ayyagar R, Xie M, et al. Comparative efficacy and safety of attention-deficit/hyperactivity disorder pharmacotherapies, including guanfacine extended release: a mixed treatment comparison. Eur Child Adolesc Psychiatry. 2017;26:875–97.
Riera M, Castells X, Tobias A, et al. Discontinuation of pharmacological treatment of children and adolescents with attention deficit hyperactivity disorder: meta-analysis of 63 studies enrolling 11,788 patients. Psychopharmacology. 2017;234(17):2657–71.
Lopez FA, Leroux JR. Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges. Atten Defic Hyperact Disord. 2013;5(3):249–65.
Hodgkins P, Shaw M, McCarthy S, et al. The pharmacology and clinical outcomes of amphetamines to treat ADHD. CNS Drugs. 2012;26(3):245–68.
Banaschewski T, Coghill D, Santosh P, et al. Long-acting medications for the hyperkinetic disorders. A systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006;15(8):476–95.
Weber J, Siddiqui MA. Lisdexamfetamine dimesylate. CNS Drugs. 2009;23(5):419–25.
Steer C, Froelich J, Soutullo C, et al. Lisdexamfetamine dimesylate. CNS Drugs. 2012;26(8):691–705.
Shire Pharmaceutical Contracts Limited. Elvanse 20, 30, 40, 50, 60 & 70 mg capsules, hard. UK summary of product characteristics 2017. https://www.medicines.org.uk/emc/product/2979/smpc. Accessed 5 June 2018.
Frampton JE. Lisdexamfetamine: a review in ADHD in adults. CNS Drugs. 2016;30(4):343–54.
Shire US inc. Vyvanse® (lisdexamfetamine dimesylate) capsules, for oral use. US prescribing information. 2015. https://www.accessdata.fda.gov. Accessed 5 June 2018.
Blick SK, Keating GM. Lisdexamfetamine. Paediatr Drugs. 2007;9(2):129–35.
Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr. 2010;6:317–27.
Najib J. Lisdexamfetamine in the treatment of adolescents and children with attention-deficit/hyperactivity disorder. Adolesc Health Med Ther. 2012;3:51–66.
Dittmann RW, Cardo E, Nagy P, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs. 2013;27(12):1081–92.
Coghill D, Banaschewski T, Lecendreux M, et al. European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23(10):1208–18.
Coghill DR, Banaschewski T, Lecendreux M, et al. Maintenance of efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: randomized-withdrawal study design. J Am Acad Child Adolesc Psychiatry. 2014;53(6):647–57.e1.
Coghill DR, Banaschewski T, Nagy P, et al. Long-term safety and efficacy of lisdexamfetamine dimesylate in children and adolescents with ADHD: a phase IV, 2-year, open-label study in Europe. CNS Drugs. 2017;31:625–38.
Newcorn JH, Nagy P, Childress AC, et al. Randomized, double-blind, placebo-controlled acute comparator trials of lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS Drugs. 2017;31:999–1014.
Shire. Effectiveness of Vyvanse compared to Concerta in adolescents with attention-deficit/hyperactivity disorder [ClinicalTrials.gov identifier NCT01552915]. US National Institutes of Health, ClinicalTrials.gov [online]. 2014. https://clinicaltrials.gov/ct2/show/NCT01552915. Accessed 5 June 2018.
Shire. Effectiveness of Vyvanse compared to Concerta in adolescents with attention-deficit/hyperactivity disorder [ClinicalTrials.gov identifier NCT01552902]. US National Institutes of Health, ClinicalTrials.gov [online]. 2015. https://clinicaltrials.gov/ct2/show/NCT01552902. Accessed 5 June 2018.
Nottinghamshire Area Prescribing Committee. Methylphenidate. Information sheet for primary care prescribers. 2016. http://www.nottsapc.nhs.uk. Accessed 5 June 2018.
Lecendreux M, Banaschewski T, Soutullo C, et al. Efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: effect of age, sex and baseline disease severity. In: 21st European Congress of Psychiatry; 2014.
Coghill DR, Banaschewski T, Lecendreux M, et al. Post hoc analyses of the impact of previous medication on the efficacy of lisdexamfetamine dimesylate in the treatment of attention-deficit/hyperactivity disorder in a randomized, controlled trial. Neuropsychiatr Dis Treat. 2014;10:2039–47.
Soutullo C, Banaschewski T, Lecendreux M, et al. A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. 2013;27(9):743–51.
Coghill DR, Banaschewski T, Lecendreux M, et al. Efficacy of lisdexamfetamine dimesylate throughout the day in children and adolescents with attention-deficit/hyperactivity disorder: results from a randomized, controlled trial. Eur Child Adolesc Psychiatry. 2014;23(2):61–8.
Banaschewski T, Soutullo C, Lecendreux M, et al. Health-related quality of life and functional outcomes from a randomized, controlled study of lisdexamfetamine dimesylate in children and adolescents with attention deficit hyperactivity disorder. CNS Drugs. 2013;27(10):829–40.
Dittmann RW, Cardo E, Nagy P, et al. Treatment response and remission in a double-blind, randomized, head-to-head study of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention-deficit hyperactivity disorder. CNS Drugs. 2014;28(11):1059–69.
Nagy P, Häge A, Coghill DR, et al. Functional outcomes from a head-to-head, randomized, double-blind trial of lisdexamfetamine dimesylate and atomoxetine in children and adolescents with attention/deficit/hyperactivity disorder and an inadequate response to methylphenidate. Eur Child Adolesc Psychiatry. 2016;25(2):141–9.
Banaschewski T, Johnson M, Lecendreux M, et al. Health-related quality of life and functional outcomes from a randomized-withdrawal study of long-term lisdexamfetamine dimesylate treatment in children and adolescents with attention deficit/hyperactivity disorder. CNS Drugs. 2014;28(12):1191–203.
Coghill D, Caballero B, Sorooshian S, et al. A systematic review of the safety of lisdexamfetamine dimesylate. CNS Drugs. 2014;28(6):497–511.
Banaschewski T, Johnson M, Nagy P, et al. Growth and puberty in a 2-year open-label study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder. CNS Drugs. 2018;32(5):455–67.
Coghill DR, Banaschewski T, Bliss C, et al. Cognitive function of children and adolescents with attention-deficit/hyperactivity disorder in a 2-year open-label study of lisdexamfetamine dimesylate. CNS Drugs. 2018;32(1):85–95.
Faraone SV, Spencer TJ, Kollins SH. Effects of lisdexamfetamine dimesylate treatment for ADHD on growth. J Am Acad Child Adolesc Psychiatry. 2010;49(1):24–32.
Goodman DW. Lisdexamfetamine dimesylate. The first prodrug stimulant. Psychiatry (Edgemont). 2007;4(8):39–45.
Faraone SV. Lisdexamfetamine dimesylate: the first long-acting prodrug stimulant treatment for attention deficit/hyperactivity disorder. Expert Opin Pharmacother. 2008;9:1565–74.
Ermer J, Adeyi B, Pucci M. Pharmacokinetic variability of long-acting stimulants in the treatment of children and adults with attention-deficit hyperactivity disorder. CNS Drugs. 2010;24(12):1009–25.
Weyandt LL, Marraccini ME, Gudmundsdottir BG, et al. Pharmacological interventions for adolescents and adults with ADHD: stimulant and nonstimulant medications and misuse of prescription stimulants. Psychol Res Behav Manag. 2014;7:223–49.
Zimovetz EA, Beard SM, Hodgkins P, et al. A cost-utility analysis of lisdexamfetamine versus atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and inadequate response to methylphenidate. CNS Drugs. 2016;30(10):985–96.
Shire. Comparison of lisdexamfetamine dimesylate with atomoxetine HCl in attention-deficit/hyperactivity disorder (ADHD) subjects with an inadequate response to methylphenidate [ClinicalTrials.gov identifier NCT01106430]. US National Institutes of Health, ClinicalTrials.gov [online]. 2014. https://clinicaltrials.gov/ct2/show/study/NCT01106430. Accessed 5 June 2018.
Acknowledgements
During the peer review process, the manufacturer of lisdexamfetamine was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
James Frampton is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: D.R. Coghill, Department of Paediatrics, Faculty of Medicine, Dentistry and Health Science, University of Melbourne, Melbourne, Australia; S. Cortese, Centre for Innovation in Mental Health, Academic Unit of Psychology, University of Southampton, Southampton, UK; R. Jain, Department of Psychiatry, Texas Tech Health Sciences Center School of Medicine, Midland, TX, USA; M. Johnson, Gillberg Neuropsychiatry Center, University of Gothenburg, Gothenburg, Sweden; J. Najib, LIU, Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Brooklyn, NY, USA; C.R. Steer, Paediatric Department, Victoria Hospital, Kirkcaldy, UK.
Rights and permissions
About this article
Cite this article
Frampton, J.E. Lisdexamfetamine Dimesylate: A Review in Paediatric ADHD. Drugs 78, 1025–1036 (2018). https://doi.org/10.1007/s40265-018-0936-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-018-0936-0