Abstract
Introduction
As chimeric antigen receptor T-cell therapies are becoming increasingly available in the armamentarium of the hematologist, there is an emerging need to monitor post-marketing safety.
Objective
We aimed to better characterize their safety profile by focusing on cytokine release syndrome and identifying emerging signals.
Methods
We queried the US Food and Drug Administration Adverse Event Reporting System (October 2017–September 2020) to analyze suspected adverse drug reactions to tisagenlecleucel (tisa-cel) and axicabtagene ciloleucel (axi-cel). Disproportionality analyses (reporting odds ratio) were performed by comparing chimeric antigen receptor T-cell therapies with (a) all other drugs (reference group 1) and (b) other onco-hematological drugs with a similar indication, irrespective of age (reference group 2), or (c) restricted to adults (reference group 3). Notoriety was assessed through package inserts and risk management plans. Adverse drug reaction time to onset and cytokine release syndrome features were investigated.
Results
Overall, 3225 reports (1793 axi-cel; 1433 tisa-cel) were identified. The reported toxicities were mainly: cytokine release syndrome (52.2%), febrile disorders (27.7%), and neurotoxicity (27.2%). Cytokine release syndrome and neurotoxicity were often co-reported and 75% of the events occurred in the first 10 days. Disproportionalities confirmed known adverse drug reactions and showed unexpected associations: for example, axi-cel with cardiomyopathies (reporting odds ratio = 2.3; 95% confidence interval 1.2–4.4) and gastrointestinal perforations (2.9; 1.2–7.3), tisa-cel with hepatotoxicity (2.5; 1.1–5.7) and pupil disorders (15.3; 6–39.1).
Conclusions
Our study confirms the well-known adverse drug reactions and detects potentially emerging safety issues specific for each chimeric antigen receptor T-cell therapy, also providing insights into a stronger role for tisa-cel in inducing some immunodeficiency-related events (e.g., hypogammaglobulinemia, infections) and coagulopathies, and for axi-cel in neurotoxicity.
Similar content being viewed by others
Chimeric antigen receptor T cells are innovative therapies for hematologic malignancies, for which a strict post-marketing monitoring has been recommended. |
Our disproportionality analyses identified new potential signals, as cardiomyopathies and gastrointestinal perforations for axi-cel, hepatotoxicity and pupil disorders for tisa-cel. |
Our findings provide insights into different safety profiles for the chimeric antigen receptor T-cell therapies investigated, and into a unified neurotoxicity-cytokine release syndrome. |
1 Introduction
Chimeric antigen receptor T (CAR-T) cells represent an innovative treatment in the hematology/oncology field. They are represented by autologous T cells, obtained through leukapheresis, engineered to express a chimeric receptor directed towards the selected tumor antigen, mostly CD-19. Upon recognition of the target, the construct induces T-cell proliferation and activation against CD19+ B cells and B-cell precursors, both normal and malignant [1, 2]. Two second-generation CAR-T products, tisagenlecleucel (tisa-cel®) and axicabtagene ciloleucel (axi-cel®), were approved by the US Food and Drug Administration [3, 4] and the European Medicines Agency [5] for the treatment of relapsed or refractory (r/r) large B-cell lymphomas, such as diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. Tisa-cel was also approved for the treatment of patients up to 25 years of age with r/r B-cell acute lymphoblastic leukemia (ALL) [6].
Although pivotal randomized controlled trials have shown extremely positive results in terms of efficacy for the two CAR-T cells, potentially life-threatening or even fatal toxicities have also been reported [7,8,9]. In particular, cytokine release syndrome (CRS) and neurotoxicity are two major complications that can lead to significant morbidity and mortality [10]. Cytokine release syndrome is a systemic inflammatory reaction associated with the release of cytokines, such as interleukin-6, interferon-γ, and tumor necrosis factor, occurring mainly in the first days after infusion. The broad spectrum of CRS-related signs includes fever, hypotension, hypoxia, depressed cardiac function, and organ dysfunction [11]. In pivotal trials, grade ≥ 3 CRS occurred in 22% of patients treated with tisa-cel and 11% of patients treated with axi-cel, with a median time to onset (TTO) of 3 days. Other adverse events reported with an incidence > 20% were hypogammaglobulinemia, infections, decreased appetite, headache, delirium, encephalopathy, bleeding episodes, nausea, diarrhea, vomiting, edema, fatigue, and acute kidney injury [7, 9].
Based on the pre-marketing assessment of their safety profile, in 2017, CAR-T cells were approved by the US Food and Drug Administration (FDA) with a Risk Evaluation and Mitigation Strategy, a program required for medications associated with serious safety concerns, as a risk minimization measure ensuring that benefits outweigh risks. In Europe, an accelerated assessment application through the Priority Medicines (PRIME) scheme for orphan diseases has been granted for CAR-T cells and additional monitoring and post-authorization safety studies have been required by the European Medicines Agency [12] after marketing authorization. Increasing post-marketing data have been recorded for CAR-T-cell safety profile monitoring in a real-world setting, where a significantly larger and heterogeneous population of patients has been treated [13,14,15,16].
In view of the several new CAR-T products recently approved by the FDA on the market (e.g., brexucabtagence autoleucel for mantle cell lymphoma in July 2020, lisocabtagepane maraleucel for DLBCL in February 2021, and idecabtagene vicleucel for multiple myeloma in March 2021), CAR-T cells are anticipated to be used in an increasing number of patients. This study aims to investigate the post-marketing safety profile of the well-established tisa-cel and axi-cel, analyzing spontaneous suspected adverse drug reaction (ADR) reports collected into the FDA Adverse Event Reporting System (FAERS) database, with the final goal to better characterize already known CAR-T-cell-related ADRs (primarily CRS) and to identify emerging safety reports of potential clinical relevance.
2 Methods
2.1 Data Source
The FAERS is a spontaneous reporting system collecting worldwide reports of suspected ADRs, submitted by heterogeneous reporters, with a range from healthcare professionals to patients and their families, lawyers, and manufacturers [17]. The FAERS data are available to the public in different ways: (a) a user-friendly public dashboard (available at the link: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard), containing many duplicates and limited information and (b) raw quarterly data downloadable as ASCII or XML files (available at the link: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html), which need to be pre-processed but allow for more reliable analyses. Suspected ADRs are coded using preferred terms (PTs) from the Medical Dictionary for Regulatory Activities (MedDRA®), which has a hierarchical structure allowing the grouping of PTs at higher levels (i.e., High-Level Terms [HLTs]; High-Level Group Terms; System Organ Class).
ASCII files from the inception up to September 2020 were downloaded, merged, and active substances were translated to an implementation of the World Health Organization drug dictionary (accessed in March 2020) [18]. Data have been pre-processed to keep only the last update of each report and to remove duplicates, i.e., reports with the same information in all the following fields: sex, age, weight, country, date of the ADR, list of drugs (i.e., either suspect or concomitant), and list of ADRs.
2.2 Exposure of Interest
Reports of suspected ADRs related to tisa-cel and axi-cel from the post-marketing setting were retrieved in the period October 2017 to September 2020. In order to exclude all CAR-T-cell reports associated with pre-marketing experimental studies, we selected only reports related to approved indications for use and with entry dates following the first approval date (Table 1). As the main comparator (reference group 1, RG1), the reports of all other drugs collected in the same study period have been considered. In order to adjust for confounding by indication, only reports related to onco-hematological drugs, with an approved indication of use for ALL or DLBCL, have been considered as a second comparator (reference group 2, RG2). To correct also for age, RG2 reports restricted to adults (aged ≥ 18 years) have been considered as an additional reference group (RG3).
2.3 Data Analysis
Descriptive analyses have been carried out to explore and compare the characteristics of CAR-T-cell reports (e.g., age and sex of patients, temporal trends in reporting, reporter types) versus RG1, RG2, and RG3, and to describe the distribution of adverse events by MedDRA® HLTs. Continuous variables were summarized as median with interquartile ranges (IQRs) and categorical variables as percentages.
The reporting odds ratio (ROR) was used as a measure of ADR reporting disproportionality for signal detection (at the HLT level), with a statistical threshold that was defined as the lower limit of 95% confidence interval >1 in presence of three or more reports [19]. Disproportionality analyses on the FAERS database have already been applied with success to the oncological field, particularly to immunotherapy [20]. To reduce the risk of detecting false associations, we also calculated the information component (IC). The IC is a Bayesian disproportionality that applies shrinkage and converts to the base 2 logarithm the ratio between observed and expected cases [21]. If IC025 is estimated less than 0, we cannot with high confidence exclude that the disproportionality may be due to random variation and small numbers. Two authors evaluated the notoriety of prioritized ADRs, retaining significance on the three RGs. We considered unexpected the disproportions with HLTs that did not include PTs already included in the specific CAR-T-cell package inserts (i.e., the US FDA summary of product characteristics). To account for the redundancy of MedDRA® terms (i.e., the same event may be recorded with different terms), we considered expected also terms linked to those included in package inserts. Finally, important potential risks as defined by the European risk management plans (RMPs) [i.e., “concerns for which an association with the CAR-T cell is possible based on available data, but this association has not been established yet and needs further evaluation”] were investigated in depth through a case-by-case assessment. These potential risks were tumor lysis syndrome (only for axi-cel), second primary neoplasia, autoimmune conditions, and graft versus host disease (GvHD). Data considered in the case-by-case assessment were those concerning the drug and the event, sex, age, outcome, and TTO.
To assess the TTO of suspected ADRs, we calculated the number of days that elapsed from the beginning of the CAR-T-cell cycle up to the onset of suspected ADRs at their System Organ Class level and for HLTs and PTs of interest. Time to onset was summarized as the median and IQR.
To investigate the co-occurrence of CRS and other ADRs, a network analysis with HLTs as nodes and relevant relationships as links was performed, by employing validated methods for psychopathological symptoms in clinical settings (Ising estimation) [22], already applied to pharmacovigilance [23]. Links’ weights were estimated as partial correlations between each pair of HLTs conditioning on all other HLTs. A LASSO (least absolute shrinkage and selection operator) procedure was performed to filter out spurious associations. Data management and analysis were performed using the packages tidyverse, datatable, ggradar, qgraph, visNetwork, and IsingFit of the “R” software (version 4.02).
3 Results
3.1 Descriptive Analysis
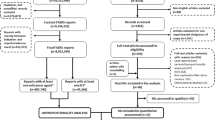
Among 3,253,919 overall reports collected in the FAERS during the period October 2017–September 2020, a total of 3225 CAR-T-cell reports from the post-marketing setting were identified: 1793 (55.6%) for axi-cel and 1433 (44.4%) for tisa-cel (in one case, both CAR-T cells were reported). Additionally, 3,250,642 reports other than CAR-T have been selected as RG1, including 142,083 reports of drugs for hematology/oncology indications (RG2) and, of these, 74,170 (52.2%) were restricted to adults (RG3) (Fig. 1). Of the 1441 axi-cel reports with a specified indication of use, 1433 (99.4%) referred to non-Hodgkin B-cell lymphoma, eight to lymphocytic leukemia. Of the 1276 tisa-cel reports with a specified indication for use, 744 (58.3%) referred to lymphocytic leukemia, and 532 (41.7%) to non-Hodgkin B-cell lymphoma.
Flow chart for identification of post-marketing reports of suspected adverse events related to chimeric antigen receptor T (CAR-T) cells in the period 1 October, 2017–30 September, 2020. FAERS FDA Adverse Event Reporting System, RG1 reference group 1 (all reports other than the ones related CAR-T cells), RG2 reference group 2 (reports related to onco-hematological drugs with indication for use specified for acute lymphoblastic leukemia or diffuse large B-cell lymphoma), RG3 reference group 3 (onco-hematological drug reports with indication for use specified for acute lymphoblastic leukemia or diffuse large B-cell lymphoma in the adult population specifically)
As compared with RG1, a higher percentage of CAR-T-cell reports regarded male patients (60.5% for axi-cel; 59.6% for tisa-cel) versus 38.7%. Among tisa-cel reports, the median age was 21 (IQR = 12–60) years, with 42.4% of reports in patients aged younger than 18 years, whereas in axi-cel it was 61 (52-68).
Chimeric antigen receptor T-cell-related ADRs were reported by medical doctors (N = 604; 35.6% for axi-cel; N = 769; 54.2% for tisa-cel) and other healthcare practitioners (N = 298; 17.6% for axi-cel; N = 203; 14.3% for tisa-cel) more often than RG1 (22.6% and 6.5%, respectively) (Table 2). A higher proportion of serious ADRs was observed for CAR-T cells (94.6%) compared with RG1 (56.2%), and RG2 (76.9%), with death accounting for 16.6% of axi-cel-related reports and 27.0% of tisa-cel-related reports (vs 8.2 in RG1 and 10.5 in RG2) (Table 2). Immune and associated conditions not elsewhere classified (NEC) [46.7%, e.g., CRS, hemophagocytic lymphohistiocytosis (HLH)], febrile disorders (35.3%), and alterations in white blood cell counts (30.8%, mostly a decrease) were the adverse events by HLTs more frequently reported for tisa-cel, while immune and associated conditions NEC (56.7%), nervous system disorders NEC (35.6%, i.e., neurotoxicity), and febrile disorders (21.7%) were the top three adverse events by HLTs reported for axi-cel. Noteworthy, neoplasm malignant site unspecified NEC-related events—more than 90% malignant neoplasm progression—were more reported for tisa-cel than for axi-cel (22.7% vs 0.9%, respectively) [see Fig. 2 and Table S1 of the Electronic Supplementary Material (ESM)].
3.2 Disproportionality Analysis
Many disproportionality signals correspond to side effects already reported in package inserts, such as immune-related conditions (i.e., CRS and HLH), immunodeficiency, and neurotoxicity (see Table 3 and Table S2–5 of the ESM). New potential signals were also identified. For axi-cel, the main disproportions in decreasing frequency of report count were bladder and urethral symptoms (ROR = 2.1, 1.5–3.1; IC = 1, 0.4–1.4; i.e., urinary incontinence), cardiomyopathies (ROR = 2.3, 1.2–4.4; IC = 1, 0–1.8) and gastrointestinal ulcers and perforation, site unspecified (ROR = 2.9, 1.2–7.3; IC = 1.2, − 0.3 to 2.2). For tisa-cel, new signals were the following: neoplasms malignant site unspecified NEC (ROR = 18.7, 15.6–22.3; IC = 3.5, 3.3–3.7; i.e., malignant neoplasm progression, second primary malignancies), metabolic acidosis (ROR = 8.2, 4.5–14.9; IC = 2.5, 1.6–3.2), paralysis and paresis (ROR = 3.6, 1.9–6.6; IC = 1.6, 0.6–2.3), hepatic failure and associated disorders (ROR = 2.5, 1.1–5.7; IC = 1.1, −0.3 to 2), and pupil disorders (ROR = 15.3, 6–39.1; IC = 2.6, 1.1–3.6).
3.3 Risk Management Plan-Driven Case-by-Case Assessment
Reports of adverse events listed in RMPs were also explored (see Tables S6–9 of the ESM):
-
Fifty cases of second primary non-hematological neoplasia were reported with axi-cel (17 reports, 0.95%) and tisa-cel (33 reports, 2.30%) versus 3.62% in RG2 and 4.37% in RG3. In 32 of these, a specific cancer was recorded (most frequently involving skin [seven cases], genitourinary tract [six cases], lung, [five cases], and the liver and pancreas [three cases]);
-
Twenty-nine cases of autoimmune conditions (mostly affecting nervous tissue [Guillain–Barre syndrome, myelitis, encephalitis] in patients aged more than 40 years, and only one case in a young patient [Crohn’s disease in a 13-year-old boy]) were reported with axi-cel (11 reports, 0.61%) and tisa-cel (18 reports, 1.26%);
-
Fourteen reports of GvHD (all with tisa-cel, 0.98%, in patients aged younger than 25 years), including one involving the lung, one the skin, one the gastrointestinal tract, and one involving the skin, liver, and gastrointestinal tract;
-
Five reports of tumor lysis syndrome with axi-cel (0.28%; two specified latencies of 8 and 2 days).
3.4 Time to Onset
Time-to-onset analysis at the System Organ Class level (see Fig. S1 of the ESM) was restricted to reports with specified TTO (the TTO was unspecified in 1527 cases, 47.3%). Overall, 75% of the events occurred within 10 days (median = 5, IQR = 2–10 days; missing = 1527/3225) from CAR-T-cell administration, with immune (3, 2–6; 765/1809), cardiovascular (3, 2–7; 124/379), and neurological (4, 2–7; 572/1526) adverse events in the first week, and infections (6, 2–21; 390/751), and neoplasia (9, 2–44; 460/709) occurring also over a longer period.
3.5 Cytokine Release Syndrome In-Depth Analysis
Immune and associated conditions NEC occurred in 1685 (52.2%) CAR-T reports, with the following specific PTs: CRS in 1657 of the reports, HLH in 59, GvHD in 14, cytokine storm in 13, and other immune system disorder in four. As compared to RG2 (to avoid excluding suspected ADRs reported in children), statistically significant disproportionate reporting was observed for HLH (ROR = 12.07, 8.13–17.93 for axi-cel; ROR = 10.77, 7.38–15.71 for tisa-cel) and GvHD (14 cases, always in tisa-cel; ROR = 2.48, 1.45–4.23). Even if 90% of CRS events occurred within the first 10 days, we also found reports of delayed CRS (> 12 days), frequently co-reported with cytopenia (14% vs 4%). Based on the network analysis, vascular hypotensive disorders, conditions associated with abnormal gas exchange, nervous system disorders NEC, and encephalopathies toxic and metabolic were commonly reported together with immune and associated conditions NEC, whereas leukopenias NEC were preferentially reported without immune and associated conditions (Fig. 3).
Network of significant relationships between chimeric antigen receptor T-induced cytokine release syndrome and other suspected adverse drug reactions. Each node represents a High-Level Term, each link a significant relationship identified through the Ising estimation of the network: green links define a covariance, while red links identify couples of mutually exclusive adverse drug reaction terms. nec not elsewhere classified
4 Discussion
Our study explored the post-marketing safety profile of tisa-cel and axi-cel through the analysis of the US FAERS database. In particular, we focused on new potential safety signals, on the comparison of the two CAR-T cells, on CRS features, and on events to be monitored according to RMPs.
4.1 Case Demographics
The demographics of the CAR-T-cell reports were in line with previous pharmacovigilance studies [16, 24, 25]. More reports from male patients were expected (channeling bias), as both DLBCL and ALL show a higher prevalence in male patients, as reported by the US cancer scientific authority [26, 27]. Furthermore, differences in age among the two CAR-T-cell reports are explained by the indication for ALL in pediatric patients exclusive for tisa-cel [6].
We also found a higher frequency of serious suspected ADRs for CAR-T cells, as compared with all other drugs in FAERS as well as versus other drugs approved for the treatment of ALL and/or DLBCL. This finding is partly explained by the fact that CAR-T cells are administered only in r/r ALL and DLBCL, and a role of the Risk Evaluation and Mitigation Strategy program recommendations to report any serious suspected ADR cannot be excluded. In particular, we observed a slightly higher proportion of fatalities as compared with a previous study carried out in FAERS (21.2% vs 15.3%) [28]. As we included also reports outside the USA, which are collected only for serious and unexpected ADRs, this finding could be a result of the different reports evaluated.
Overall, the first 10 days following CAR-T-cell administration is the time window at the highest risk for developing serious ADRs. In line with a previous study performed in Vigibase [25], CRS and neurologic adverse events were reported to occur within the first week after CAR-T-cell administration.
4.2 New Potential Safety Signals
Our study confirmed well-known safety issues (e.g., CRS, cytopenia, neurotoxicity, rate and rhythm disorders) that have been reported in pivotal clinical trials as well as observational studies [7,8,9, 14, 24, 25, 29], and their management has been extensively addressed in the recent American Society of Clinical Oncology guidelines for the management of immune-related ADRs in patients treated with CAR-T-cell therapy [30]. However, we found also new potential signals concerning ADRs that have not been described in the package insert. While disproportionate reporting of splenic and lymphatic disorders (e.g., lymphadenopathy and splenomegaly) with tisa-cel are plausibly related to its use in ALL, and can be explained as confounding by indication, cholestasis/jaundice and hepatic failure, metabolic disorders, lower respiratory tract signs and symptoms, and hallucinations represent new potential safety signals.
Previous studies found transaminases increase during CAR-T-cell administration, usually together with CRS [11, 31,32,33], whereas we found 16 cases of hepatic failure with tisa-cel. The fact that CRS was concomitantly recorded in all but three of these reports suggests that liver injury was plausibly a manifestation of CRS-mediated organ dysfunction, and not a drug-induced direct hepatotoxicity.
We also found a potential association with pupil disorders (i.e., pupils unequal, pupil fixed, and mydriasis) that could lead to visual impairment as already reported in the package insert (7% of treated patients in clinical trials) and to a higher contribution of visual disorders by tisa-cel observed in a recently published FAERS analysis [34].
Axi-cel, instead, was found to be also associated with gastrointestinal infarctions, hemorrhages, ulcers, and perforations. Accordingly, in a clinical study on the long-term effects of CAR-T cells, around 2% of patients died because of duodenal ulcer and perforation [35]. Nonetheless, it should be noted that tumor lysis of gastrointestinal lymphomas in this type of patient (listed as an important potential risk according to the RMP of axi-cel) could be partly responsible for these findings.
Furthermore, axi-cel was found to be associated with cardiomyopathies, in accordance with previous observational studies [13, 36]. It is important to note that, as shown by the negative IC025, we cannot exclude that the significant RORs for tisa-cel and hepatic failure, and for axi-cel and gastrointestinal ulcers and perforation were due to random variation and small numbers.
4.3 Potential Differences in Tisagenlecleucel and Axicabtagene Ciloleucel Safety Profiles
Potential differences in tisa-cel and axi-cel safety profiles emerged from our findings. In particular:
-
1.
a stronger role for tisa-cel compared with axi-cel in inducing immunodeficiency-related disorders (especially infections) was supported by:
-
(a)
the higher proportion of reports describing fever (35.3 vs 21.7%), white blood cell count decrease (30.8 vs 2.9%), and immunodeficiency disorders NEC (17.1 vs 2.0%);
-
(b)
the stronger disproportionality with immunodeficiency disorders NEC even when limiting the analysis to adults (in RG3 ROR = 35.7, 28.7–44.4 vs 2.2, 1.4–3.4; IC = 4.4, 4.1–4.6 vs 1, 0.4–1.5);
-
(c)
the exclusive association, in RG3, with many infections, particularly by opportunistic agents (e.g., Aspergillus, Candida, fungi, Klebsiella).
-
(a)
-
2.
a stronger role for tisa-cel compared with axi-cel in inducing coagulopathies was supported by:
-
(a)
the stronger association with coagulopathies (in RG3 ROR = 9.9, 6.6–14.8 vs 2.6, 1.6–4.3; IC = 2.9, 2.3–3.4 vs 1.3, 0.4–1.8);
-
(b)
the exclusive association with thrombocytopenias and coagulation factor deficiencies, also potentially associated with the already discussed hepatic failure;
-
(c)
the exclusive association with cerebrovascular accidents (even if IC025 < 0) and plausibly related events (e.g., paresis).
-
(a)
-
3.
a stronger role for axi-cel compared with tisa-cel–in inducing neuropsychiatric ADRs, supported by:
-
(a)
the higher proportion of reports describing nervous system disorders NEC (35.6 vs 16.7%);
-
(b)
a stronger association with neuropsychiatric events (e.g., in RG3 nervous system disorders NEC ROR = 76.3, 66.6–87.3 vs 17.4, 14.1–21.3; IC = 4.6, 4.5–4.7 vs 3.6, 3.3–3.8);
-
(c)
the exclusive association with many neurological events (i.e., memory loss, speech and language disorders, mental impairment, autonomic nervous system disorders, encephalitis NEC, thinking disturbances, communications disorders);
-
(d)
the exclusive association with incontinence, which is plausibly a symptom of severe neurotoxicity.
-
(e)
the consistency with results from a previous study performed on the FAERS public dashboard, in December 2019 [14].
-
(a)
The only neuro-psychiatric HLTs significantly associated with tisa-cel but not with axi-cel were the already mentioned vascular events, autoimmune conditions (myelitis and Guillain–Barré syndrome), and hallucinations.
4.4 Risk Management Plan-Driven Case-by Case Assessment
We found 50 cases of CAR-T cells related to second primary non-T-cell/non-hematological neoplasia (mostly skin, genitourinary, and lung cancer). Patients with non-Hodgkin lymphoma have a known higher risk for developing secondary malignancies [37, 38], as supported also by the comparable reporting proportion of non-hematological malignancies in both RG2 and RG3; therefore, the role of the underlying clinical condition of these patients cannot be excluded. Accordingly, ten reports of second primary non-hematological neoplasia occurred less than 1 month after CAR-T administration, thus pointing towards no causal association. Nonetheless, second primary malignancies are listed in the RMPs as plausible adverse reactions to CAR-T. In fact, the treatment with CAR-T cells could unmask and speed up the progression of pre-existing neoplasms, considering that it targets exclusively CD19-positive cells, therefore being ineffective on other malignancies. Moreover, the immunosuppression associated with the CAR-T treatment could be another favoring factor to be taken into account.
Twenty-nine reports of autoimmune, mostly neurological, conditions have also been identified, with a preferential association with tisa-cel. The hypothesized relationship between CAR-T cells and immune-related ADRs requires further assessment.
Graft versus host disease is another potential risk listed in the CAR-T-cell RMPs. Chimeric antigen receptor T-cell administration may have a role in aggravating pre-existing GvHD in relapsed patients after prior allogeneic hematopoietic stem cell transplantation, even if results from clinical trials are heterogeneous [8, 33, 39]. In FAERS, we identified 14 GvHD reports that occurred after tisa-cel administration, involving multiple anatomical sites (i.e., skin, liver, gastrointestinal tract, lung).
4.5 Cytokine Release Syndrome Features
Immune and associated conditions NEC occurred in 52.2% of CAR-T-cell reports: mainly as CRS and other overlapping terms (e.g., cytokine storm [40]), but also as hemophagocytic lymphohistiocytosis (considered a particularly severe form of CRS). The profile of CRS emerging from our analysis is in line with the literature, occurring around day 3 from the injection, and manifesting together with abnormal gas exchange and hypotension. Our findings, furthermore, point to a unified CRS-neurotoxicity framework, for the following reasons:
-
(a)
the frequent co-reporting of CRS, neurotoxicity, and encephalopathies;
-
(b)
a common TTO, limited mostly to the first week;
-
(c)
a high proportion of neurotoxicity co-reported with CRS (in 30% of CRS cases it occurred within 10 days following the CAR-T injection, and in 36% of CRS cases it occurred after 10 days).
Indeed, CAR-T-cell-induced neurotoxicity occurs almost exclusively in patients experiencing also CRS, of any grade [41]. However, few reports of neurotoxicity occurring after resolution of CRS, or without CRS, have been recorded, suggesting the co-existence also of an independent pathogenic mechanism [42]. Interestingly, we found that 8% of CRS reports occurred after at least 12 days after the infusion (delayed CRS [43]).
4.6 Limitations and Strengths
In consideration of the observational nature of our study on FAERS, well-known limitations of these studies are related to the analysis of spontaneous reporting systems, including data missingness (e.g., disease burden and cancer site) and a lack of information on the exposed population (i.e., total patients treated with CAR-T cells). Moreover, it must be taken into account that CAR-T cells have been approved for the treatment of advanced disease, after the failure of several previous lines of treatment. Therefore, we cannot exclude that disproportionate reporting of some adverse events could be determined also by the effects of the treatments administered before CAR-T as well as comorbidities and the underlying clinical status of these heavily pretreated patients. The large-scale design of our study, while accounting for age and indication confounders, did not allow us to correct for biases affecting individual suspect ADRs. These biases should be considered in studies focused on individual drug-event combinations.
Our work relies on and underlines the important strengths of pharmacosurveillance that make it complementary to pivotal trials: the access to a population usually neglected in pre-marketing studies, a large population with reports from all over the world and an extended focus to any type of event. Moreover, we designed and performed disproportionality analyses taking into account potential biases and carefully scrutinizing potential signals, in relation to the information reported in the package insert and RMPs. The analysis performed at the HLT level allows the grouping of some of the MedDRA® terms with overlapping meanings (with higher numbers and accuracy), but at the cost of sometimes grouping not overlapping terms (e.g., hypernatremia and hyponatremia). We also performed RMP-driven individual case assessments to search for potential risks not optimally retrieved using an HLT. Finally, we exploited the richness of pharmacosurveillance data to better characterize CAR-T-related ADRs, particularly considering TTO and co-reporting of events.
5 Conclusions
The present analysis provides an overview of spontaneous reports of suspected ADRs occurring in patients treated with CAR-T cells. Our results strengthen the evidence provided by clinical trials and previous observational studies. We also found unexpected signals that are worthy of further evaluation, including gastrointestinal perforations and cardiomyopathies for axi-cel and hepatic failure and pupil disorders for tisa-cel. The higher frequency of reporting of non-hematologic second primary neoplasia does not support theory-based RMP alerts, pointing instead to the role of the underlying disease; nonetheless, further studies are needed to elucidate a possible additional contribution by CAR-T-cell therapy. Finally, we provided insights into a stronger role for tisa-cel in inducing immunodeficiency-related events (i.e., hypogammaglobulinemia and susceptibility to infections) and coagulopathies, and for axi-cel in neurotoxicity, and into a unified CRS-neurotoxicity syndrome.
References
Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4.
Hartmann J, Schüßler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9:1183–97.
US FDA. FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma. Accessed 26 Jun 2022.
US FDA. FDA approves axicabtagene ciloleucel for large B-cell lymphoma. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-large-b-cell-lymphoma. Accessed 26 Jun 2022.
European Medicines Agency. First two CAR-T cell medicines recommended for approval in the European Union. 2018. https://www.ema.europa.eu/en/news/first-two-car-t-cell-medicines-recommended-approval-european-union. Accessed 26 Jun 2022.
US FDA. FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-b-cell-all-and-tocilizumab-cytokine-release-syndrome. Accessed 26 Jun 2022.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy: assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30.
Elsallab M, Levine BL, Wayne AS, Abou-El-Enein M. CAR T-cell product performance in haematological malignancies before and after marketing authorisation. Lancet Oncol. 2020;21:e104–16.
Alvi RM, Frigault MJ, Fradley MG, Jain MD, Mahmood SS, Awadalla M, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T). J Am Coll Cardiol. 2019;74:3099–108.
Gajra A, Zettler ME, Phillips EG, Klink AJ, Jonathan KK, Fortier S, et al. Neurological adverse events following CAR T-cell therapy: a real-world analysis. Immunotherapy. 2020;12:1077–82.
Rafaniello C, Ferrajolo C, Gaio M, Zinzi A, Scavone C, Sullo MG, et al. Tisagenlecleucel in children and young adults: reverse translational research by using real-world safety data. Pharmaceuticals (Basel). 2020;13:258.
Bonaldo G, Montanaro N, Alberto V, Motola D. Safety profile of chimeric antigen receptor T-cell immunotherapies (CAR-T) in clinical practice. Eur J Clin Pharmacol. 2021;77:1225–34.
Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013;10:796–803.
Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data mining techniques in pharmacovigilance: analysis of the publicly accessible fda adverse event reporting system (AERS). In: Karahoca A., editor. Data mining applications in engineering and medicine [Internet]. London: IntechOpen; 2012. Available from: https://www.intechopen.com/chapters/38579. https://doi.org/10.5772/50095.
Reynolds RF, Kurz X, de Groot MCH, Schlienger RG, Grimaldi-Bensouda L, Tcherny-Lessenot S, et al. The IMI PROTECT project: purpose, organizational structure, and procedures. Pharmacoepidemiol Drug Saf. 2016;25:5–10.
Raschi E, Gatti M, Gelsomino F, Ardizzoni A, Poluzzi E, De Ponti F. Lessons to be learnt from real-world studies on immune-related adverse events with checkpoint inhibitors: a clinical perspective from pharmacovigilance. Target Oncol. 2020;15:449–66.
Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22:57–69.
van Borkulo CD, Borsboom D, Epskamp S, Blanken TF, Boschloo L, Schoevers RA, et al. A new method for constructing networks from binary data. Sci Rep. 2014;4:5918.
Fusaroli M, Raschi E, Gatti M, De Ponti F, Poluzzi E. Development of a network-based signal detection tool: the COVID-19 Adversome in the FDA Adverse Event Reporting System. Front Pharmacol. 2021;12:3542.
Guha A, Addison D, Jain P, Gutierrez JM, Ghosh A, Roddie C, et al. Cardiovascular events associated with chimeric antigen receptor T cell therapy: cross-sectional FDA Adverse Events Reporting System analysis. Biol Blood Marrow Transplant. 2020;26:2211–6.
Dolladille C, Ederhy S, Ezine E, Choquet S, Nguyen LS, Alexandre J, et al. Chimeric antigen receptor T-cells safety: a pharmacovigilance and meta-analysis study. Am J Hematol. 2021;96:1101–11.
National Cancer Institute, SEER. Diffuse large B-cell lymphoma: cancer stat facts. 2021. https://seer.cancer.gov/statfacts/html/dlbcl.html. Accessed 2 Nov 2021.
National Cancer Institute, SEER. Acute lymphocytic leukemia: cancer stat facts. 2021. https://seer.cancer.gov/statfacts/html/alyl.html. Accessed 2 Nov 2021.
Zettler ME, Feinberg BA, Phillips EG, Klink AJ, Mehta S, Gajra A. Real-world adverse events associated with CAR T-cell therapy among adults age ≥ 65 years. J Geriatr Oncol. 2021;12:239–42.
Miao L, Zhang Z, Ren Z, Li Y. Reactions related to CAR-T cell therapy. Front Immunol. 2021;12:1501.
Santomasso BD, Nastoupil LJ, Adkins S, Lacchetti C, Schneider BJ, Anadkat M, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J Clin Oncol. 2021;9(35):3978–92.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18.
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–20.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28.
Fischer A, Li A. CAR T-cell therapy associated ocular adverse events reported to the Food and Drug Administration. Invest Ophthalmol Vis Sci. 2020;61:3636.
Cordeiro A, Bezerra ED, Hill JA, Turtle CJ, Maloney DG, Bar M. Late effects of CD19-targeted CAR-T cell therapy. Blood. 2018;132:223.
Ganatra S, Redd R, Hayek SS, Parikh R, Azam T, Yanik GA, et al. Chimeric antigen receptor T-cell therapy-associated cardiomyopathy in patients with refractory or relapsed non-Hodgkin lymphoma. Circulation. 2020;142:1687–90.
Smeland KB, Kiserud CE, Lauritzsen GF, Blystad AK, Fagerli U-M, Falk RS, et al. A national study on conditional survival, excess mortality and second cancer after high dose therapy with autologous stem cell transplantation for non-Hodgkin lymphoma. Br J Haematol. 2016;173:432–43.
Tward JD, Wendland MMM, Shrieve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:108–15.
Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. OncoImmunology. 2015;4: e1027469.
Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–73.
Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183:364–74.
Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433–44.
Hay KA, Hanafi L-A, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306.
Acknowledgements
MF, ER, and EP were supported by institutional research funds (Ricerca Fondamentale Orientata).
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest/Competing Interests
MF, VI, PC, CF, FD, VC, and EP declare that they have no conflicts of interest. ER reports a personal fee for consultancy from Novartis, outside the submitted work. GT has served on advisory boards, seminars, masters, and projects funded by several pharmaceutical companies (Eli Lilly, Amgen, Sanofi, SOBI, Gilead, PTC Therapeutics, ABBvie, Verpora, Daiichi Sankyo), outside the submitted work.
Ethics Approval
Anonymized data were collected from a publicly available database and do not require ethics committee approval.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available in the US FAERS repository.
Code Availability
The R script developed for the analyses is available from the corresponding author upon reasonable request.
Authors’ Contributions
MF, VI, PC, CF, ER, FD, VC, ER, EP, and GT conceived and executed the research project. MF designed and executed the statistical analysis. MF, VI, PC, CF, ER, EP, and GT reviewed and critiqued the statistical analysis. All the authors contributed to the interpretation of data. Regarding article preparation, the writing of the first draft was executed by MF and VI, and all the authors contributed to the review and critique. All the authors approved the final version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fusaroli, M., Isgrò, V., Cutroneo, P.M. et al. Post-Marketing Surveillance of CAR-T-Cell Therapies: Analysis of the FDA Adverse Event Reporting System (FAERS) Database. Drug Saf 45, 891–908 (2022). https://doi.org/10.1007/s40264-022-01194-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01194-z