Abstract
Background and Objectives
Idarucizumab is an antibody fragment that specifically reverses dabigatran-mediated anticoagulation. Safety, pharmacokinetics and pharmacodynamics of idarucizumab were investigated in dabigatran-treated, middle-aged, elderly and renally impaired volunteers with characteristics similar to patients receiving anticoagulant therapy.
Methods
In this randomized, double-blind, crossover study, 46 subjects (12 middle-aged, 45–64 years; 16 elderly, 65–80 years; and 18 with mild or moderate renal impairment) received dabigatran etexilate (DE; 220 or 150 mg twice daily) for 4 days. Idarucizumab doses of 1, 2.5 and 5 g or 2 × 2.5 g 1 h apart, or placebo, were administered as a rapid (5 min) infusion ~2 h after DE at steady state.
Results
Dabigatran-prolonged diluted thrombin time, ecarin clotting time and activated partial thromboplastin time were reversed to baseline immediately after idarucizumab infusion in all groups. Reversal was sustained with doses ≥2.5 g. Idarucizumab was well tolerated under all conditions. No impact of age on idarucizumab pharmacokinetics was observed; however, subjects with mild or moderate renal impairment demonstrated increased exposure (up to 84 %), decreased clearance and prolonged (by up to 49 %) initial half-life of idarucizumab compared with healthy middle-aged subjects.
Conclusions
Impaired renal function was associated with increased exposure and decreased clearance of idarucizumab. Idarucizumab resulted in immediate, complete and sustained reversal of dabigatran anticoagulant activity, and was safe and well tolerated in middle-aged, elderly and renally impaired volunteers. The results support the clinical use of a 5 g dose of idarucizumab.
Clinical Trial Registration
http://www.clinicaltrials.gov. Unique identifier: NCT01955720.
Similar content being viewed by others
These data expand on existing observations in young, healthy volunteers by showing that idarucizumab (a humanized monoclonal antibody fragment) immediately reverses the anticoagulant effect of dabigatran in middle-aged, elderly and renally impaired volunteers. |
Efficacy of idarucizumab in renally impaired subjects was observed, together with an increase in exposure and reduced clearance of idarucizumab in this group. |
An ongoing phase III study, RE-VERSE AD™, is assessing the effect of idarucizumab 5 g on dabigatran-induced anticoagulation in emergency patients with uncontrolled bleeding or who require urgent surgery or procedures. |
1 Introduction
Dabigatran etexilate (DE) is the oral prodrug of dabigatran, a direct thrombin inhibitor, which is effective for the prevention and treatment of thromboembolic events in patients with atrial fibrillation [1–7]. In a retrospective analysis, the outcomes of major and life-threatening bleeding events with DE were favorable when compared with warfarin [8]. While these bleeding events are uncommon, a specific, fast-acting reversal agent for dabigatran would provide a further treatment option when immediate reversal of anticoagulation is needed [9].
Idarucizumab, a humanized monoclonal antibody fragment, neutralizes dabigatran activity in a 1:1 stoichiometric relationship, with a very high binding affinity [10]. Previous studies demonstrated that intravenous (5 min) infusion of idarucizumab resulted in immediate, complete and sustained reversal of dabigatran-induced anticoagulation in young, healthy male volunteers, and was safe and well tolerated [11, 12].
Elderly and renally impaired patients represent a large proportion of the atrial fibrillation population [1, 13] and a reversal agent needs to be effective and safe in these patient subgroups. Moreover, patients with atrial fibrillation who need to undergo urgent surgical procedures, or have an uncontrolled bleeding event, will require re-initiation of anticoagulation after reversal to prevent stroke. Thus, these reversal agents should have short half-lives so that it is possible to re-start anticoagulation within a reasonable time frame.
The present study examines the pharmacodynamics (PD) of the reversal of dabigatran anticoagulant effect by idarucizumab in volunteers who are elderly or have some degree of renal impairment (RI). In addition, the effects of age and renal function on the pharmacokinetics (PK) of idarucizumab are investigated.
2 Methods
2.1 Study Design and Subjects
The study (NCT01955720) was performed at SGS Life Science Services, Antwerp, Belgium, from September 2013 to August 2014. The protocol was approved by an independent Ethics Committee and the trial was carried out in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice and applicable regulatory requirements.
Four subject groups were recruited: middle-aged (45–64 years), elderly (65–80 years), RI: 60–90 (aged 45–80 years with mild RI, defined as creatinine clearance [CrCl] ≥60 to <90 mL/min using the Cockcroft–Gault equation at the screening visit), and RI: 30–60 (aged 45–80 years with moderate RI, defined as CrCl ≥30 to <60 mL/min). All subjects were healthy, except for the groups with compromised renal function, who were in relatively good health in all other respects. Inclusion criteria for body mass index were 18.5–29.9 kg/m2 for middle-aged subjects, or 18.5–32 kg/m2 for elderly and RI subjects. All subjects provided written informed consent.
The study was divided into three treatment periods, two of which are reported in this study (Fig. 1), and the third reported elsewhere [14]. In the first treatment period, all four groups were treated orally with DE on days 1–3, with a single dose on day 4, and then randomized within each group to either idarucizumab or placebo. Study treatment was blinded for the investigator and subjects and those assessing outcomes. Blood sampling occurred at multiple time points on day 4 before and after idarucizumab/placebo infusion, and for up to 120 h post-infusion. Thus, treatment period 1 lasted 8 days (extended to 10 days for the RI groups).
Treatment period 2 occurred after a group completed treatment period 1, with at least a 6-day washout. Period 2 was the same as period 1, except volunteers now received the opposite treatment as prespecified in the crossover design. Additionally, in the middle-aged group only, subjects were retreated with DE 24 h after idarucizumab/placebo infusion during periods 1 and 2 (data reported elsewhere [14]).
The third treatment period (results reported elsewhere [14]) applied only to the middle-aged group, who underwent re-exposure with idarucizumab (after 4 days of treatment with DE, idarucizumab on day 4), 2 months after completing treatment period 2. Idarucizumab was administered in doses of 1, 2.5 and 5 g or 2 × 2.5 g 1 h apart, as outlined in Fig. 1. All idarucizumab doses were administered as a 5-min intravenous infusion beginning 1 h and 55 min after the final DE dose. Idarucizumab, matching placebo and DE (Pradaxa®) capsules were provided by Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach an der Riß, Germany.
2.2 Endpoints and Analyses
The anticoagulant effect of dabigatran and its reversal by idarucizumab were assessed by measuring diluted thrombin time (dTT), ecarin clotting time (ECT), activated partial thromboplastin time (aPTT) and thrombin time in a central laboratory. On site, activated clotting time was measured by the iSTAT point-of-care device. The primary analysis was the percentage of subjects with reversal of dabigatran anticoagulation, measured as dTT and ECT within 10 min after idarucizumab infusion (after the second idarucizumab 2.5 g infusion for the RI: 30–60 mL/min group). Secondary analyses included reversal of dabigatran-induced anticoagulation assessed by aPTT; reversal of mean dTT, ECT and aPTT in each group; and reduction of area under the dabigatran-effect curve (AUEC above baseline coagulation time) for coagulation parameters.
Reversal was defined as a clotting time returning to or below a threshold level. All available data from this study and a previous study were combined to determine these thresholds [11, 12], which were calculated via a linear regression model correlating concentrations of unbound dabigatran with the clotting parameters dTT and ECT (supplementary Fig. 1; Online Resource). For dTT, ECT and aPTT, the value corresponds with 20 ng/mL unbound dabigatran, a plasma level with presumably low anticoagulant effect. Complete reversal was defined as the reduction of mean coagulation time to below the threshold, immediate reversal was the complete reversal directly after idarucizumab infusion, and sustained reversal was mean coagulation time remaining below the threshold during the full observation period of ≥24 h.
The AUEC further quantified the anticoagulant effect of dabigatran over time. Determination of AUECs took into account the baseline clotting times, i.e. the AUECs above baseline dTT, ECT and aPTT were calculated. The AUEC from 2 to 12 h after DE administration (AUEC2–12) in volunteers receiving placebo was compared with the values obtained after idarucizumab dosing for dTT, ECT and aPTT. The mean ratio of AUEC2–12 of idarucizumab to placebo quantified the reversal effect of idarucizumab over the first 10 h after its administration versus placebo. Plasma concentrations of total dabigatran, unbound dabigatran and idarucizumab were assessed at predetermined timepoints. Total dabigatran was defined as bound plus unbound dabigatran, including its active metabolites in plasma, while unbound dabigatran was the fraction that was bound to neither idarucizumab nor plasma proteins and was an approximate measure of pharmacologically active dabigatran (supplementary Fig. 1; Online Resource).
PK parameters for idarucizumab include the area under the plasma concentration–time curve from time zero to infinity (AUC ∞ ), maximum measured plasma concentration (C max), initial half-life (t ½,2, describing the initial decline of the plasma concentration immediately after C max), terminal half-life (t ½), total clearance (CL), volume of distribution at steady state (V ss) and volume of distribution during the terminal phase (V z ). The fraction of idarucizumab eliminated in urine from 0 to 6 h (fe6) was also assessed.
The primary safety endpoint was the number (%) of subjects with drug-related adverse events (AEs; i.e. related to DE, idarucizumab or placebo) [see Sect. 1.2 Safety Assessment; Online Resource].
2.3 Pharmacological Methods
Blood samples for PK and PD analysis were taken at scheduled timepoints on days 1, 4, 5, 6 and 7 (and days 8 and 9 for subjects with RI). PK samples (K3-EDTA vials) and PD samples for dTT, ECT and aPTT samples (3.2 % Na-Citrate vials) were centrifuged to obtain plasma. Urine sampling for PK analysis was performed over discrete time intervals until 26, 74 and 122 h after the last intake of DE for middle-aged, elderly and renally impaired subjects, respectively.
Analyses of dTT (Hemoclot; Hyphen BioMed, Neuvillesur Oise, France), ECT (in-house assay, 6 U/mL ecarin; Pentapharm, Basel, Switzerland) and aPTT (CK Prest; Diagnostica Stago, Asnières-sur-Seine, France) were performed using validated assays [15] at Menal GmbH, Emmendingen, Germany (see Sect. 1.1 Pharmacological Analyses; Online Resource). Adequate assay performance during study sample analysis was demonstrated by analysis of quality control (QC) samples. Mean assay imprecision (% coefficient of variation) was ≤7.5 % for the Hemoclot dTT assay (n = 128 for each of three dabigatran concentration levels), ≤3.3 % for the aPTT assay (n = 150, single level) and ≤3.0 % for the ECT assay (n = 144, single level) over the course of the study sample analysis.
Validated enzyme-linked immunosorbent assay methods were used to determine idarucizumab concentrations in plasma and urine, with a lower limit of quantification (LLOQ) of 1 µg/mL in both matrices. Analyses were performed at Covance Laboratories, Inc., Chantilly, VA, USA. Adequate assay performance during analysis of study samples was demonstrated by the results of QC samples. For the plasma QC (n = 118 for each of the three levels of QC and n = 74 for the dilution QC), mean accuracy (% deviation from nominal concentration) was within ±5.2 %, and mean precision (% coefficient of variation) was <17.6 %. For the urine QC (n = 66 for each of the three levels of QC and n = 40 for the dilution QC), mean accuracy was within ±9.1 % and mean precision was <=20.2 %.
Concentrations of total and unbound dabigatran in plasma were determined by validated high-performance liquid chromatography–tandem mass spectrometry assays at Nuvisan GmbH, Neu-Ulm, Germany. The LLOQs were 5.00 ng/mL for total dabigatran and 1.00 ng/mL for unbound dabigatran. Adequate assay performance during study sample analysis was demonstrated by analysis of QC samples (three concentrations per analyte, n = 62 each). For total dabigatran, mean accuracy (% deviation from nominal concentration) was within ±5.3 %, and mean precision (% coefficient of variation) was ≤5.1 %. For unbound dabigatran, mean accuracy and precision were within ±3.8 and ≤8.1 %, respectively.
2.4 Statistical Evaluation
Descriptive statistics for PD and PK endpoints were calculated. Safety was also evaluated descriptively. Correlation between unbound dabigatran and anticoagulation parameters was assessed using linear least squares regression analysis. All PD data are expressed as mean ± standard error of the mean, and all PK data are expressed as the geometric mean unless otherwise stated. A post hoc analysis of PK parameters of AUC∞, t ½,2 and CL, using two sample t tests, was undertaken to evaluate potential differences between the groups receiving idarucizumab 5 g, relative to the middle-aged volunteer group.
3 Results
3.1 Subject Disposition and Baseline Characteristics
A total of 46 middle-aged, elderly and RI (RI: 60–90 and RI: 30–60) volunteers were included and received study treatment. All subjects completed the trial and were included in the PK, PD and safety analyses. Demographic and baseline characteristics were similar across subgroups, except for age and renal function (Table 1). Mean weight was slightly lower in the RI: 30–60 group.
3.2 Dabigatran Anticoagulation in the Absence of Idarucizumab
Upon placebo treatment, unbound dabigatran was ~30 % less than total dabigatran, which is accounted for by dabigatran binding to plasma proteins. Unbound dabigatran is considered to approximate pharmacologically active dabigatran (supplementary Fig. 1; Online Resource).
In middle-aged volunteers receiving DE 220 mg twice daily, peak steady-state levels of ~170 ng/mL unbound dabigatran were achieved ~2 h after final DE ingestion. This was associated with elevations of approximately 1.8-fold of dTT, ~3-fold of ECT and ~2.2-fold of aPTT over their respective baseline values (Fig. 2a–c). Plasma levels of unbound dabigatran decreased to below the LLOQ early after idarucizumab administration, and remained <20 ng/mL for the 24 h observation period (Fig. 2d). In elderly volunteers, peak unbound dabigatran plasma levels were slightly higher, 210 ng/mL (Fig. 3d). This was associated with a higher level of anticoagulation than in middle-aged volunteers, with approximately 2.1-, 3.6-, and 2.2-fold elevations of dTT, ECT and aPTT, respectively, over baseline values (Fig. 3a–c). In RI: 60–90 and RI: 30–60 subjects receiving DE 150 mg twice daily, peak unbound dabigatran plasma levels were ~140 ng/mL (Fig. 4d) and ~220 ng/mL (Fig. 5d), respectively, reflecting the dependency of dabigatran elimination on renal function. Prolongation of clotting times correlated with dabigatran concentration, with greater prolongation in RI: 30–60 subjects than RI: 60–90 subjects (Figs. 4a–c, 5a–c).
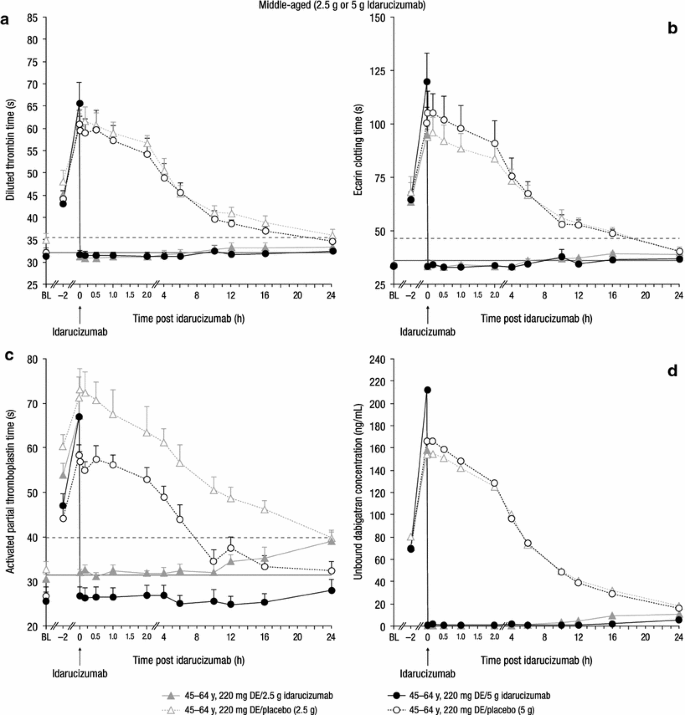
Middle-aged subjects. Mean-effect time profiles (+SEM) of standard clotting assays (a–c) and geometric mean concentration–time profile of unbound dabigatran (d) at dabigatran steady state after infusion of idarucizumab 2.5 or 5 g, or placebo. Solid line (a–c) represents mean BL; dashed line (a–c) represents the threshold for complete reversal; ‘0’ on the x-axis, end of idarucizumab infusion. BL baseline, DE dabigatran etexilate, SEM standard error of the mean
Elderly subjects. Mean-effect time profiles (+SEM) of standard clotting assays (a–c) and geometric mean concentration–time profile of unbound dabigatran (d) at dabigatran steady state after infusion of idarucizumab 1 or 5 g, or placebo. Solid line (a–c) represents mean BL; dashed line (a–c) represents the threshold for complete reversal; ‘0’ on the x-axis, end of idarucizumab infusion. BL baseline, DE dabigatran etexilate, SEM standard error of the mean
RI: 60–90 subjects. Mean-effect time profiles (+SEM) of standard clotting assays (a–c) and geometric mean concentration–time profile of unbound dabigatran (d) at dabigatran steady state after infusion of idarucizumab 1 or 5 g, or placebo. Solid line (a–c) represents mean BL; dashed line (a–c) represents the threshold for complete reversal; ‘0’ on the x-axis, end of idarucizumab infusion. RI renal impairment, BL baseline, DE dabigatran etexilate, SEM standard error of the mean
RI: 30–60 subjects. Mean-effect time profiles (+SEM) of standard clotting assays (a–c) and geometric mean concentration–time profile of unbound dabigatran (d) at dabigatran steady state after infusion of idarucizumab 2 × 2.5 g or matching placebo. Solid line (a–c) represents mean BL; dashed line (a–c) represents the threshold for complete reversal; ‘0’ on the x-axis, end of idarucizumab infusion. RI renal impairment, BL baseline, DE dabigatran etexilate, SEM standard error of the mean
3.3 Idarucizumab Administration: Effect of Age and Renal Function on Idarucizumab Pharmacokinetics and on the Reversal of Dabigatran Anticoagulation
3.3.1 Middle-Aged Volunteers, Aged 45–64 Years
Idarucizumab PK (Table 2; Fig. 6a, c) were characterized by AUC∞ of 37,000 nM·h/L and a C max of 25,000 nmol with the idarucizumab 5 g dose. The subsequent plasma concentration–time profile showed a rapid initial decline and a slower terminal phase. The initial half-life of 47 min accounted for most of the idarucizumab AUC; the terminal phase started ~6 h after infusion, with plasma levels reduced to ~2 % of C max. The V ss was 8.86 L and CL was 47.1 mL/min. PK profiles were similar in males and females (data not shown).
Geometric mean concentration–time profiles of idarucizumab plasma concentration following administration of idarucizumab 5 g at dabigatran steady state in middle-aged, elderly and RI: 60–90 subjects on a linear (a) and semi-log (c) scale. Plasma concentrations of total and unbound dabigatran and idarucizumab following administration of idarucizumab 5 g at dabigatran steady state in middle-aged subjects on a linear (b) and semi-log scale (d). Geometric mean concentration–time profiles of total and unbound dabigatran and idarucizumab in subjects with RI: 30–60 are available in supplementary Fig. 5 (Online Resource)
Idarucizumab administration of 2.5 or 5 g resulted in immediate reduction of the dabigatran-prolonged coagulation times to baseline levels (i.e. below the threshold value). This effect was sustained for the entire subsequent observation period of 24 h. Figure 2a–c illustrates the reversal of all three coagulation parameters with both doses of idarucizumab compared with placebo. Similar results were obtained with thrombin time and activated clotting time data, as summarized in supplementary Table 1 and supplementary Figs. 3 and 4 (Online Resource). The effect was associated with a decrease of unbound dabigatran concentrations to <1 ng/mL (LLOQ) immediately after idarucizumab administration; concentrations remained below or very close to the LLOQ for the entire observation period of 24 h (Fig. 2d). Mean plasma concentrations of total dabigatran (bound plus unbound fraction) increased ~4.5- and 5.2-fold over pre-infusion levels within ~30 min after idarucizumab infusion of 2.5 and 5 g, respectively, which is consistent with dabigatran binding and inactivation by idarucizumab in the plasma (data shown for 5 g in Fig. 6b, d). Thereafter, levels of total dabigatran declined to <50 ng/mL at 24 h.
Dabigatran anticoagulation was also quantified as the AUEC over 10 h after idarucizumab or placebo administration (AUEC2–12) (Table 3). Administration of idarucizumab 2.5 and 5 g reduced the AUEC2–12 by >95 % for both dTT and ECT, and by >86 % for aPTT (Table 3). No dose dependency was observed; in middle-aged volunteers, the maximum effect was already achieved with idarucizumab 2.5 g.
3.3.2 Elderly Volunteers, Aged 65–80 Years
The idarucizumab 5 g dose in elderly volunteers was associated with AUC∞ of 43,900 nM·h/L, C max of 28,300 nmol and t ½,2 of 55 min (Table 2). In comparison with the middle-aged volunteer group, there were no significant differences in AUC∞ (p = 0.1089), t½,2 (p = 0.0787) or CL (p = 0.1089). The plasma concentration–time profile in elderly volunteers was very similar to that of middle-aged volunteers (Fig. 6).
Idarucizumab doses of 1 or 5 g resulted in immediate and complete reversal of anticoagulation (Fig. 3a–c). Complete reversal of anticoagulation was sustained over the observation period of 72 h with the 5 g dose, whereas a partial return of dabigatran anticoagulation was measured 2 h after idarucizumab 1 g. The AUEC2–12 was reduced dose-dependently, by >63 % for dTT, ECT and aPTT with the 1 g dose, and by >94 % for the idarucizumab 5 g dose (Table 3).
Similar to findings in middle-aged volunteers, unbound dabigatran concentrations decreased to <1 ng/mL (LLOQ) immediately after idarucizumab 1 or 5 g, and remained low for the 72-h observation period with the higher dose (Fig. 3d). Plasma concentrations of total dabigatran increased approximately fivefold over pre-infusion levels of 270 ng/mL within 30 min of idarucizumab dosing.
3.3.3 Mild Renal Impairment (RI) [RI: 60–90]
The idarucizumab 5 g dose in the RI: 60–90 volunteers was associated with an increased AUC∞ of 53,100 nM·h/L compared with that in middle-aged volunteers (p = 0.002), and a C max of 32,100 nmol, achieved at the end of the idarucizumab infusion (Table 2). In the initial phase, plasma concentrations declined with a t½,2 of 56 min, similar to elderly volunteers, and prolonged compared with middle-aged volunteers (p = 0.0099). CL was reduced compared with middle-aged volunteers (p = 0.002) (Table 2). At the start of the terminal phase at ~6 h, idarucizumab concentrations had declined to <3 % of C max (Fig. 6).
Idarucizumab administration of 1 or 5 g resulted in an immediate, dose-dependent reduction of dabigatran-prolonged clotting times to baseline values (Fig. 4a–c), similar to findings in elderly volunteers. The AUEC2–12 of dTT and ECT after idarucizumab (1 and 5 g doses) was reduced by >76 and >98 %, respectively, and the AUEC2–12 of aPTT by >63 % with 1 g and >98 % with 5 g (Table 3).
As seen in elderly subjects, unbound dabigatran concentrations also decreased to <1 ng/mL after idarucizumab 1 or 5 g, with the effect sustained at the high dose (Fig. 3d). Total dabigatran levels were approximately 200 ng/mL prior to idarucizumab dosing, and increased following idarucizumab to ~5.5-fold within ~30 min, and then declined, as seen in the other groups.
3.3.4 Moderate RI (RI: 30–60)
Administration of 2 × 2.5 g idarucizumab in the RI: 30–60 volunteers was associated with an AUC∞ of 67,900 nM·h/L and a Cmax of 25,600 nmol, achieved at the end of the second infusion (Table 2). Idarucizumab exposure (AUC∞) in this group was increased by 83.5 % relative to the middle-age group receiving idarucizumab 5 g (p < 0.0001). C max cannot be directly compared with the other groups due to the split dose (supplementary Fig. 5; Online Resource). The initial half-life of approximately 70 min was prolonged relative to the other groups (extended by 48.7 % relative to the middle-age group receiving idarucizumab 5 g; p = 0.0003) (Table 2). The terminal phase started when plasma concentrations had already declined to low levels (i.e. ~7 % or less of Cmax), and was similar to the other groups. CL was 25.7 mL/min in the RI: 30–60 volunteers compared with 47.1 mL/min in the middle-aged group (p < 0.0001) [Table 2].
Volunteers with moderate RI received a total dose of idarucizumab 5 g, administered as two 5-min infusions of 2.5 g 1 h apart. After the first infusion, there was an immediate reduction of anticoagulation to baseline values with all assays tested, and these remained reduced after the second infusion (Fig. 5a–c). The AUEC2–12 of dTT, ECT and aPTT after idarucizumab infusion was reduced by >98 % (Table 3).
Unbound dabigatran decreased to <1 ng/mL (LLOQ) in the RI: 30–60 group after the first infusion, and remained decreased over the measurement period of 120 h (Fig. 5d). Prior to idarucizumab dosing, total dabigatran levels were 278 ng/mL. Concentrations of total dabigatran increased 5.8-fold over pre-infusion levels within 30 min and then declined following the first idarucizumab infusion (supplementary Fig. 5b; Online Resource). The second idarucizumab infusion had no further effect on total dabigatran concentrations.
3.4 Urinary Excretion of Idarucizumab
In all treatment groups and with all idarucizumab doses, the largest portion of idarucizumab excreted in urine was between 0 and 6 h post-infusion, with negligible amounts present in subsequent collection intervals. The fraction of idarucizumab dose excreted in urine (fe6) following administration of a total dose of idarucizumab 5 g ranged between 30.0 and 39.8 % across volunteer groups. Fe6 was dose-dependent; for example, an increase from 9.4 to 39.8 % was observed following administration of idarucizumab 1 and 5 g to elderly volunteers (Table 2).
3.5 Safety and Tolerability
Overall, 31 subjects (67 %) reported AEs during the trial: 21 (46 %) during pretreatment with dabigatran, 14 (30 %) while receiving idarucizumab, and 12 (26 %) while receiving placebo. All AEs were of mild intensity; no AEs were serious, led to discontinuation, or were indicative of allergic or immunogenic reactions. AE frequency did not increase with idarucizumab dose, age or stage of RI. During the treatment period and follow-up, AEs defined as possibly drug-related by the investigator were reported for six subjects (13 %) [see Sect. 2.1 Safety; Online Resource).
4 Discussion
In male and female volunteers aged 45–80 years with normal or reduced renal function (CrCl > 30 mL/min), idarucizumab resulted in immediate and complete reversal of dabigatran-induced anticoagulation. The effect was sustained for the entire observation period of at least 24 h with doses of 2.5 or 5 g. Impaired renal function reduced idarucizumab clearance, thereby increasing its AUC and prolonging its initial half-life. However, idarucizumab was cleared rapidly, with <7 % of the maximal concentration measureable after 6 h. In line with the mode of action, reversal of dabigatran anticoagulation occurred in parallel with the reduction of unbound dabigatran plasma concentrations. All doses of idarucizumab were safe and well tolerated.
Reversal of dabigatran anticoagulation by idarucizumab was independent of age and renal function. Elderly (mean age 69 years) and middle-aged subjects (mean age 52 years) had complete and sustained reversal of dabigatran anticoagulation with the idarucizumab 5 g dose, demonstrated using dTT, ECT and aPTT and supported by measurement of unbound dabigatran concentrations. Similarly, reversal was independent of impaired renal function, demonstrated by the complete and sustained reversal observed in volunteers with mean CrCl of 76 and 59 mL/min in the RI: 60–90 and RI: 30–60 groups, respectively. Reversal effects on anticoagulation were dose dependent, with the subtherapeutic idarucizumab 1 g dose showing a partial return of anticoagulation, in contrast to the 2.5 or 5 g doses. The reversal profiles were comparable to those previously observed in younger volunteers with a mean age of 31 years and median CrCl of 130.4 mL/min [11]. This demonstrates efficacy of 2.5 g or more of idarucizumab in reversal of dabigatran anticoagulation, irrespective of age and renal function tested.
Dabigatran is predominantly cleared renally [16]. Consistent with this, dabigatran plasma levels and the degree of anticoagulation were elevated in elderly and reduced renal function volunteers. Median plasma levels at peak were similar or elevated compared with plasma levels measured in patients in RE-LY® (supplementary Fig. 2; Online Resource), indicating that reversal with idarucizumab was assessed in the setting of clinically relevant levels of dabigatran.
Overall, the PK of idarucizumab were consistent with the observations of a previous study [12]. The volume of distribution at steady state was approximately two to three times the plasma volume, indicating low tissue penetration. Initial half-life increased with decreasing renal function, which may explain the slight increase in initial half-life in elderly subjects, who also had reduced CrCl compared with middle-aged subjects (83.3 and 110.4 mL/min, respectively). Subjects with moderate and mild RI (CrCl 58.7 and 72.8 mL/min, respectively) had greater idarucizumab exposure than middle-aged subjects (p < 0.0001 and p = 0.002, respectively) due to decreased clearance (p < 0.0001 and p = 0.002, respectively) and prolonged initial half-life (p = 0.0003 and p = 0.0099, respectively) of idarucizumab. Dabigatran exposure is also increased with RI due to increased half-life [16]. The increased exposure to idarucizumab may have compensated for the increased exposure to dabigatran, and thereby ensured the complete reversal of dabigatran’s anticoagulant activity in these subjects.
The rapid and high-affinity binding of idarucizumab to dabigatran is demonstrated by the immediate decrease in unbound dabigatran and reduction of dabigatran anticoagulation. In parallel with the reduction of anticoagulant activity and consistent with the mechanism of action of idarucizumab, an increase in total dabigatran was observed. Equimolar concentrations of idarucizumab and dabigatran are required for complete neutralization of dabigatran [10, 11]. Since idarucizumab remains predominantly in blood, it binds any dabigatran in that compartment, creating a concentration gradient so that dabigatran moves from the periphery into blood. This continues until all dabigatran is bound in blood by idarucizumab, as seen with the idarucizumab 2.5 or 5 g dose, or until idarucizumab is saturated but not all dabigatran is bound, as seen with the idarucizumab 1 g dose. In plasma, unbound dabigatran was not detectable as long as the molar concentration of idarucizumab was higher than the molar concentration of total dabigatran. For the exposures of dabigatran, and corresponding dabigatran body loads achieved in this study, both idarucizumab 2.5 and 5 g were sufficient doses for sustained reversal. This illustrates that idarucizumab 5 g is in excess of that required to neutralize mean dabigatran levels seen in RE-LY, and that it does so consistently across all age groups and all levels of renal function tested. This is also the dose of idarucizumab currently being tested in a phase III clinical study [17]. Due to the rapid elimination of idarucizumab, anticoagulation treatment may then be restarted as soon as 24 h after idarucizumab administration [14].
In general, idarucizumab was safe and well tolerated; there were no AEs that resulted in discontinuation of study medication, and no clinically relevant safety concerns in any safety parameters measured.
Limitations of this study include the relatively small number of subjects assessed. A phase III study, RE-VERSE AD, is currently ongoing to assess the effect of idarucizumab at a dose of 5 g on the anticoagulant effect of dabigatran in patients who have either uncontrolled bleeding requiring urgent medical intervention, or who require emergency surgery or procedures necessitating rapid reversal of the anticoagulant effect of dabigatran [17].
5 Conclusions
In male and female volunteers with age and renal function more reflective of atrial fibrillation patients, a dose of idarucizumab 2.5 or 5 g led to immediate, complete and sustained reversal of dabigatran-induced anticoagulation. Age, sex and renal function had no impact on the reversal of dabigatran-induced anticoagulation by idarucizumab. Idarucizumab was safe and well tolerated in all subject groups. Clinical development is ongoing to assess the effect of idarucizumab on dabigatran-induced anticoagulation in patients requiring urgent reversal.
References
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Schulman S, Eriksson H, Kakkar A, et al. Influence of age and renal function on efficacy and safety of dabigatran versus warfarin for the treatment of acute venous thromboembolism: a pooled analysis of RE-COVER™ and RE-COVER™ II. Blood. 2014;121:594.
Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52.
Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–72.
Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–64.
Seeger JD, Bykov K, Bartels DB, et al. Safety and effectiveness of dabigatran and warfarin in routine care of patients with atrial fibrillation. Thromb Haemost. 2015;114:1277–89.
Villines T, Schnee J, Fraeman K, et al. The comparative safety and effectiveness of the oral anticoagulant (OAC) dabigatran versus warfarin utilized in a large healthcare system in non-valvular atrial fibrillation (NVAF) patients. Circulation. 2014;130(Suppl 2):A18353.
Majeed A, Hwang HG, Connolly SJ, et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation. 2013;128:2325–32.
Sarich TC, Seltzer JH, Berkowitz SD, et al. Novel oral anticoagulants and reversal agents: considerations for clinical development. Am Heart J. 2015;169:751–7.
Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–62.
Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. Lancet. 2015;386:680–90.
Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;113:943–51.
Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129:961–70.
Glund S, Stangier J, van Ryn J, et al. Re-starting dabigatran etexilate 24 hours after reversal with idarucizumab and re-dosing idarucizumab in healthy volunteers. J Am Coll Cardiol. 2016;67:1653–9.
Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23:138–43.
Stangier J, Rathgen K, Stahle H, et al. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–68.
Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Boehringer Ingelheim Pharma GmbH & Co. KG. Medical writing assistance, supported financially by Boehringer Ingelheim Pharma GmbH & Co. KG, was provided by PAREXEL during the preparation of this article.
Conflicts of interest
Stephan Glund, Joachim Stangier, Joanne van Ryn, Michael Schmohl, Viktoria Moschetti, Dietmar Gansser, Benjamin Lang and Jörg Kreuzer are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. Wouter Haazen was the principal investigator of this trial and is an employee of SGS Life Science Services, the contract research organization that was funded by Boehringer Ingelheim Pharma GmbH & Co. KG to perform the clinical trial. Marina De Smet is an employee of SCS Boehringer Ingelheim. Stephen Norris and Paul Reilly are employees of Boehringer Ingelheim Pharmaceuticals, Inc.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Glund, S., Stangier, J., van Ryn, J. et al. Effect of Age and Renal Function on Idarucizumab Pharmacokinetics and Idarucizumab-Mediated Reversal of Dabigatran Anticoagulant Activity in a Randomized, Double-Blind, Crossover Phase Ib Study. Clin Pharmacokinet 56, 41–54 (2017). https://doi.org/10.1007/s40262-016-0417-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-016-0417-0