Abstract
Background
Edoxaban, an oral direct factor Xa inhibitor, is in development for thromboprophylaxis, including prevention of stroke and systemic embolism in patients with atrial fibrillation (AF). P-glycoprotein (P-gp), an efflux transporter, modulates absorption and excretion of xenobiotics. Edoxaban is a P-gp substrate, and several cardiovascular (CV) drugs have the potential to inhibit P-gp and increase drug exposure.
Objective
To assess the potential pharmacokinetic interactions of edoxaban and 6 cardiovascular drugs used in the management of AF and known P-gp substrates/inhibitors.
Methods
Drug-drug interaction studies with edoxaban and CV drugs with known P-gp substrate/inhibitor potential were conducted in healthy subjects. In 4 crossover, 2-period, 2-treatment studies, subjects received edoxaban 60 mg alone and coadministered with quinidine 300 mg (n = 42), verapamil 240 mg (n = 34), atorvastatin 80 mg (n = 32), or dronedarone 400 mg (n = 34). Additionally, edoxaban 60 mg alone and coadministered with amiodarone 400 mg (n = 30) or digoxin 0.25 mg (n = 48) was evaluated in a single-sequence study and 2-cohort study, respectively.
Results
Edoxaban exposure measured as area under the curve increased for concomitant administration of edoxaban with quinidine (76.7 %), verapamil (52.7 %), amiodarone (39.8 %), and dronedarone (84.5 %), and exposure measured as 24-h concentrations for quinidine (11.8 %), verapamil (29.1 %), and dronedarone (157.6 %) also increased. Administration of edoxaban with amiodarone decreased the 24-h concentration for edoxaban by 25.7 %. Concomitant administration with digoxin or atorvastatin had minimal effects on edoxaban exposure.
Conclusion
Coadministration of the P-gp inhibitors quinidine, verapamil, and dronedarone increased edoxaban exposure. Modest/minimal effects were observed for amiodarone, atorvastatin, and digoxin.
Similar content being viewed by others
1 Introduction
Edoxaban is a novel, oral anticoagulant that inhibits factor Xa (FXa), which is located at the confluence of the intrinsic and extrinsic coagulation pathways, the primary site of amplification in the coagulation cascade [1]. Edoxaban binds to both free FXa and FXa within the prothrombinase complex, therefore producing a dose-dependent decrease in thrombin generation [2]. Edoxaban has recently been approved in Japan for prophylaxis against deep vein thrombosis in patients following hip and knee replacement surgery [3]. In addition, ongoing phase 3 trials are assessing the efficacy and safety of edoxaban for the treatment and prevention of recurrences of venous thromboembolism (Hokusai-VTE [4]) and prevention of stroke and systemic embolic events in patients with atrial fibrillation (ENGAGE-AF TIMI 48 [5]). The Hokusai-VTE trial is evaluating edoxaban 60 mg once daily, while the ENGAGE-AF trial is evaluating edoxaban 60 and 30 mg once daily [4, 5]. Edoxaban 60 and 30 mg once-daily doses were selected for ENGAGE-AF based upon a phase 2 dose-finding study in atrial fibrillation (AF) patients that showed that these 2 dosing regimens provided similar or less frequent bleeding than standard warfarin therapy [6].
The pharmacokinetics (PK) of edoxaban have been extensively studied in healthy volunteers. Edoxaban is rapidly absorbed with peak concentrations observed at 1–2 h postdose and elimination is biphasic with a mean terminal elimination half-life (t½) of 8.75–10.4 h [7]. Edoxaban is primarily eliminated unchanged through multiple pathways, with approximately 50 % of systemically absorbed drug eliminated via renal excretion. The most abundant metabolites (M4 and M1) are formed through hydrolysis with minor contribution from cytochrome P450 (CYP) 3A [8].
P-glycoprotein (P-gp) is an efflux transporter primarily expressed in the apical/luminal membrane of epithelia of the small intestine, hepatocytes, renal proximal tubules, and other sites. With broad substrate specificity and high transport capacity, P-gp can limit the systemic exposure of various xenobiotics by decreasing intestinal absorption and increasing renal excretion and biliary excretion [9–11]. Strong P-gp inhibitors may increase systemic absorption and decrease elimination of P-gp substrates, resulting in increased exposure. The US Food and Drug Administration (FDA) now recommends that all investigational drugs should be evaluated for effect on potential P-gp activity [12]. Results from transporter studies using Caco-2 cells and wild-type versus P-gp knockout mice indicate that edoxaban is a substrate for P-gp, but not for other commonly tested uptake transporters (eg, the organic anion transporter 1) [13]. Modeling and simulation analyses, which include AF patients from a phase 2 dose-finding study, have demonstrated that concomitant edoxaban and strong P-gp inhibitors increase edoxaban exposure and the risk of bleeding [14]. Therefore, it is important to assess the effect of P-gp inhibition on edoxaban PK by drugs that would be commonly co-prescribed in the AF population. The objectives of the 6 studies described here were to evaluate potential PK interactions between edoxaban and cardiovascular drugs that are known P-gp substrates (digoxin, atorvastatin, quinidine, and verapamil) and/or inhibitors (quinidine, digoxin, amiodarone, dronedarone, verapamil, and atorvastatin) and which may be prescribed to patients with AF [11, 15–18].
2 Methods
2.1 Study Designs
The design of the quinidine, verapamil, atorvastatin, and dronedarone studies were based on standard drug-drug interaction designs using 2-period, 2-treatment crossover designs and taking into consideration the disposition of edoxaban and the interacting drug [12]. Given amiodarone’s 58-day terminal elimination half-life (t1/2) [19], a single-sequence design was selected to prevent carryover. The study with digoxin used a dual-sequence, parallel design [20]. Sample sizes were based on results from previous PK studies of edoxaban and variability of edoxaban PK parameters (eg, Ogata et al. [7]). The dose of edoxaban was 60 mg, the highest dose being tested in phase 3 clinical studies. The highest recommended therapeutic doses were employed for all potentially interacting drugs. Studies were conducted at Celerion clinical research units in Neptune, NJ (quinidine, digoxin, amiodarone, verapamil, dronedarone), or Belfast, Northern Ireland, UK (atorvastatin). An institutional review board approved all protocols and each subject signed an informed consent prior to study entry. The studies were conducted in accordance with the international conference harmonisation guideline E6 for good clinical practice.
2.1.1 Quinidine
This was an open-label, randomized, 2-period, 2-treatment crossover study in 42 healthy volunteers. Subjects were randomized to receive 1 of 2 treatment regimens during the first period, followed by a washout of 7–10 days before the alternate treatment was administered during the second period. The treatments were a single, oral dose of quinidine 300 mg (Mutual Pharmaceutical Co.) on day 1, quinidine 300 mg three times daily on days 2–3, and a single dose of quinidine 300 mg on day 4, plus a single dose of edoxaban 60 mg on day 3; or edoxaban 60 mg once daily on days 1–4 and a single dose of quinidine 300 mg on day 3.
2.1.2 Digoxin
This was an open-label, dual-treatment sequence, parallel study in 48 healthy subjects assigned to digoxin (Lanoxin, GlaxoSmithKline) on days 1–7 (0.25 mg every 12 h on days 1–2, then 0.25 mg once daily on days 3–7) followed by coadministration of digoxin 0.25 mg once daily with edoxaban 60 mg once daily on days 8–14; or edoxaban 60 mg once daily on days 1–7 followed by coadministration of edoxaban 60 mg daily with digoxin on days 8–14 (0.25 mg every 12 h on days 8–9, then 0.25 mg once daily on days 10–14) [20].
2.1.3 Amiodarone
This was an open-label, single-sequence study in 30 healthy volunteers. Subjects received 2 treatments in a fixed sequence: (1) a single, oral dose of edoxaban 60 mg on day 1, then (2) amiodarone 400 mg (Pacerone, Upsher-Smith Laboratories, Inc.) once daily for 4 days (days 4–7) plus a single oral dose of edoxaban 60 mg on day 7. There was a 3-day washout period between the 2 treatments.
2.1.4 Verapamil
This was an open-label, randomized, 2-period, 2-treatment crossover study in 34 healthy volunteers. Subjects were randomized to receive one of either 2 treatment regimens during the first period, followed by a washout of at least 7 days before the alternate treatment was administered during the second period. The treatments were verapamil 240 mg sustained-release (Calan SR, Pfizer Inc.) once daily on days 1–11 and edoxaban 60 mg on day 10; or edoxaban 60 mg once daily on days 1–4 and verapamil 240 mg sustained-release on day 3.
2.1.5 Atorvastatin
This was an open-label, randomized, 2-period, 2-treatment crossover study in 32 healthy volunteers. Subjects were randomized to receive one of either 2 treatment regimens during the first period, followed by a washout of at least 7 days before the alternate treatment was administered during the second period. The treatments were atorvastatin 80 mg (Lipitor, Pfizer) once daily on days 1–8 plus edoxaban 60 mg on day 7; or edoxaban 60 mg on day 1.
2.1.6 Dronedarone
This was an open-label, randomized, 2-period, 2-treatment crossover study in 34 healthy volunteers. Subjects were randomized to receive one of either 2 treatment regimens during the first period, followed by a washout of at least 7 days before the alternate treatment was administered during the second period. The treatments were edoxaban 60 mg on day 1 under fed conditions; or dronedarone 400 mg (Multaq, Sanofi) twice daily on days 1–7 under fed conditions plus edoxaban 60 mg on day 5 under fed conditions.
2.2 Blood Sampling and Analysis for Edoxaban
Blood samples were collected to determine plasma edoxaban concentrations at serial time points over 24 h (quinidine, digoxin, and verapamil) or 72 h (amiodarone, atorvastatin, and dronedarone) after administration of edoxaban. Plasma edoxaban concentrations were measured by a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method with calibration ranges of 0.764 to 382 ng/mL (Advion BioServices, Ithaca, NY) for all studies except for the digoxin study, which had a lower limit of quantitation of 1 ng/mL.
Blood samples were also collected to confirm exposure for the potentially interacting drugs. Validated LC-MS/MS methods were used to quantify plasma drug concentrations (quinidine and atorvastatin at PPD, Richmond, VA; amiodarone at Bioanalytical Systems, Inc, West Lafayette, IN; verapamil and dronedarone at Celerion, Lincoln, NE). Digoxin was analyzed by PPD using a validated radioimmunoassay procedure.
2.3 Clinical Laboratory Assessments
Samples for routine laboratory testing were collected and tested by a Clinical Laboratory Improvement Amendments-approved laboratory. Results of hematology, serum chemistry, coagulation, urinalysis, and fecal occult blood (FOB) testing were classified according to the clinical laboratory reference ranges.
2.4 Pharmacokinetic and Statistical Analyses
Pharmacokinetic parameters calculated from the individual plasma concentrations included area under the plasma concentration versus time curve (AUC) from time 0 to the last measurable concentration or to 24 h (AUClast or AUC0–24); AUC from the time of dosing extrapolated to infinity, calculated as (AUC0–inf); maximum observed plasma drug concentration (Cmax); time of maximum observed concentration (tmax); terminal t1/2, calculated as ln(2)/λz; and mean concentrations 24 h postdose (C24). All PK parameters were calculated using WinNonlin Professional version 5.2 (Pharsight Corp., Mountain View, CA) or version 4.0 (digoxin study only). Edoxaban PK parameters and plasma concentrations were summarized using descriptive statistics.
The PK parameters for the interacting drugs were calculated when possible and summarized along with the plasma drug concentrations. All statistical summaries and analyses were performed using SAS version 8.2 (SAS Institute Inc., Cary, NC) or version 9.1.3 (dronedarone study only).
Comparisons of the PK of edoxaban administered with a potentially interacting drug versus edoxaban alone were performed using an analysis of variance (ANOVA) model with sequence, treatment, and period as fixed effects, and subject nested within sequence as a random effect. The ratios of the geometric least squares means (LSM) for the ln-transformed parameters Cmax, AUClast, and/or AUC0–inf were calculated for edoxaban alone versus edoxaban administered with the interacting drug. The 90 % confidence intervals (CI) of ratios were also calculated. If the 90 % CI was within the 80 to 125 % bioequivalence limit, it was concluded that there was no significant interaction between edoxaban and the coadministered drug.
2.5 Safety
Safety endpoints included vital signs, electrocardiograms (ECGs), adverse events (AEs), hematology, and serum chemistry.
3 Results
3.1 Subjects
In all, 220 subjects were enrolled in these studies. Demographic and baseline characteristics (Table 1) were similar across studies; most subjects were predominantly African American males with an approximate mean age of 30 years, with the exception of the study with atorvastatin, conducted in Belfast, Northern Ireland, UK, in which subjects were slightly younger (mean age 24 years) and predominantly white. At least 88 % of the subjects completed their studies with the exception of the quinidine study, where only 29 of the 42 randomized subjects completed the study (69 %): 2 were withdrawn due to protocol violations, 6 discontinued due to AEs, and 5 withdrew consent.
3.2 Pharmacokinetics
3.2.1 Edoxaban
Edoxaban administered alone resulted in a range of mean [±standard deviation (SD)] Cmax values of 223.4 (±75.0) to 295.6 (±83.6) ng/mL across the 6 studies, indicating consistent exposure of edoxaban (Tables 2, 3). Mean total exposure values for edoxaban alone expressed as AUC0–inf ranged from mean (±SD) values of 1576.8 (±322.7) to 1965.5 (±420.9) ng·h/mL, consistent across study cohorts and with historical data [7, 21–23]. In the studies with quinidine and verapamil, a 24-h collection period allowed only AUC0–24 calculations, and the mean values reported for these 2 studies showed similar exposure (1449 and 1438 ng·h/mL, respectively). Peak edoxaban concentrations were observed within 1–3 h postdose.
3.2.2 Quinidine Study
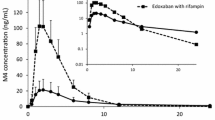
The PK parameters for edoxaban administered with and without quinidine are presented in Table 2. The geometric LSM ratio values for AUC0−24 and Cmax of edoxaban plus quinidine versus edoxaban alone were 176.7 % (90 % CI, 165.0–189.2) and 185.4 % (90 % CI, 164.8–208.5), respectively (Fig. 1); increases of 76.7 and 85.4 %, respectively. Coadministration of quinidine increased the 24-h concentration by 11.8 % (Fig. 2), and the rate of edoxaban absorption was not affected by quinidine (median tmax = 1.48 h for edoxaban alone and 1.50 h for edoxaban plus quinidine). The mean terminal elimination t1/2 values were 6.43 and 4.97 h for edoxaban alone and when administered with quinidine, respectively.
3.2.3 Digoxin Study
The geometric LSM ratio values for AUC0–τ and Cmax of edoxaban plus digoxin versus edoxaban alone were 109.5 % (90 % CI, 102.0–117.5) and 115.6 % (90 % CI, 102.8–130.0), respectively (Fig. 1); increases of 9.5 and 15.6 %, respectively [20]. Coadministration of digoxin decreased the 24-h edoxaban concentration by 9.4 % (Fig. 2). Similar rates of absorption, determined by median tmax values (1.00 and 1.50 hours, respectively) and terminal elimination t1/2 values (9.83 and 8.35 h, respectively), were observed for edoxaban alone and edoxaban plus digoxin (Table 2) [20].
3.2.4 Amiodarone Study
The PK parameters for edoxaban with and without amiodarone are listed in Table 2. The geometric LSM ratio values for AUC0–inf and Cmax of edoxaban plus amiodarone versus edoxaban alone were 139.8 % (90 % CI, 134.1–145.7) and 166.0 % (90 % CI, 146.3–188.4), respectively (Fig. 1); increases of 39.8 and 66.0 %, respectively. Coadministration of amiodarone reduced the 24-h edoxaban concentration by 25.7 % (Fig. 2). The median tmax values were 1.25 h with edoxaban alone and 1.50 h with concomitant amiodarone. The mean terminal elimination t1/2 values for edoxaban administered alone and with amiodarone were 12.25 and 10.81 h, respectively.
3.2.5 Verapamil Study
The PK parameters for edoxaban administered with and without verapamil are presented in Table 3. The geometric LSM ratios for AUC0–24 and Cmax of edoxaban plus verapamil versus edoxaban alone were 152.7 % (90 % CI, 140.9–165.4) and 153.3 % (90 % CI, 133.7–175.8) respectively (Fig. 1); increases of 52.7 and 53.3 %, respectively. Coadministration of verapamil increased the 24-h edoxaban concentration by 29.1 % (Fig. 2). The median tmax values were 1.48 h for edoxaban alone and 1.00 h for edoxaban plus verapamil. Similar terminal elimination t1/2 values were also observed for edoxaban alone (7.43 h) and edoxaban plus verapamil (6.79 h).
3.2.6 Atorvastatin Study
The PK parameters for edoxaban administered with and without atorvastatin are presented in Table 3. The geometric LSM ratio values for AUC0–inf and Cmax of edoxaban plus atorvastatin versus edoxaban alone were 101.7 % (90 % CI, 95.7–108.1) and 85.8 % (90 % CI, 76.8–95.8), respectively (Fig. 1); an increase of 1.7 % and a decrease of 14.2 %, respectively. Coadministration of atorvastatin increased the 24-h edoxaban concentration by 7.9 % (Fig. 2). The median tmax values were 1.02 h for edoxaban alone and 1.50 h for edoxaban plus atorvastatin. Similar terminal elimination t1/2 values were also observed for edoxaban alone (12.02 h) and edoxaban plus atorvastatin (12.77 h).
3.2.7 Dronedarone Study
The PK parameters for edoxaban administered with and without dronedarone are presented in Table 3. The geometric LSM ratio values for AUC0–inf and Cmax of edoxaban plus dronedarone versus edoxaban alone were 184.5 % (90 % CI, 177.9–191.4) and 145.8 % (90 % CI, 136.0–156.2), respectively (Fig. 1); increases of 84.5 and 45.8 %, respectively. Coadministration of dronedarone increased the 24-h edoxaban concentration by 157.6 % (Fig. 2). The median tmax values were 1.98 h for edoxaban alone and 2.03 h for edoxaban plus dronedarone. Similar terminal elimination t1/2 values were also observed for edoxaban alone (10.2 h) and edoxaban plus dronedarone (12.8 h).
3.3 Safety
Edoxaban was well tolerated when administered alone or with either quinidine, digoxin, amiodarone, verapamil, atorvastatin, or dronedarone. Table 4 summarizes the rates of treatment-emergent AEs (TEAEs) for each study. There were no deaths or serious AEs reported in these studies. One subject was withdrawn from the amiodarone study due to localized cellulitis. No subject withdrew prematurely from the atorvastatin study. One subject was discontinued prematurely from the digoxin study due to AEs that included symptoms of chest discomfort and AV block was noted on the ECG following digoxin treatment. Two subjects discontinued prematurely from the dronedarone study after elevated alanine aminotransferase levels were noted during coadministration of dronedarone and edoxaban. A total of 6 subjects were withdrawn from the quinidine study due to AEs, which included 2 subjects with positive FOB, 1 subject who experienced QTc prolongation after quinidine dosing, 1 subject with low hemoglobin, 1 subject with an increase in creatine kinase, and 1 subject with headache and nausea. Four subjects were withdrawn from the verapamil study due to AEs, which included 1 subject with positive FOB and 3 subjects with abnormal ECG following verapamil monotherapy.
4 Discussion
These studies explored the drug-drug interactions between edoxaban and 6 cardiovascular drugs that are likely to be commonly co-prescribed with edoxaban in the AF patient population and also have P-gp inhibition potential. These drugs display differing degrees of P-gp inhibition, with verapamil, quinidine, dronedarone, and amiodarone recognized as strong P-gp inhibitors [12], while digoxin and atorvastatin are recognized P-gp substrates [12, 16]. Coadministration of verapamil, quinidine, or dronedarone resulted in >50 % increases in total exposure measured as AUC and increases in the 24-h concentrations (a surrogate for trough concentrations) of edoxaban. Edoxaban coadministered with amiodarone increased total exposure by <50 % and 24-h concentrations decreased by approximately 26 %. Coadministration of either digoxin or atorvastatin had relatively minor effects on the PK of edoxaban.
The 90 % CI for the geometric LSM ratio values for AUC0–inf and Cmax of edoxaban were calculated per the standard test statistics described in regulatory guidelines. Salazar and colleagues used a pharmacometric model that predicted the effect of edoxaban exposure on bleeding to determine whether coadministration with these drugs would result in clinically meaningful increases in edoxaban exposure [14]. Pharmacometric analysis of a phase 2 study in patients with AF determined that trough plasma concentrations were the most robust predictors of bleeding risk [6]. Therefore, the observed edoxaban concentrations from the drug-drug interaction studies were modeled to predict trough concentrations at steady state. Increased exposure resulting in bleeding greater than the established limit (ie, greater than the observed warfarin bleeding) was considered clinically significant. On the basis of the results of the pharmacometric analysis [14], verapamil, quinidine, and dronedarone were determined to have the potential for clinically meaningful effects on the disposition of edoxaban. Therefore, a dose reduction of 50 % has been recommended for coadministration of edoxaban with verapamil, quinidine, and dronedarone. The limited effects of atorvastatin and digoxin on edoxaban PK may be due to their weaker affinity for the P-gp transporter; therefore, these drugs, at therapeutic doses, are not considered strong P-gp inhibitors for edoxaban. Previous publications have cited verapamil, quinidine, amiodarone, and dronedarone as potent P-gp inhibitors based on the >25 % increase in AUC of digoxin, considered a probe substrate for P-gp [12, 16, 24–26]. Although amiodarone has been shown to have strong P-gp inhibitory effects with other P-gp substrates, the increased edoxaban exposure was modest. Based on the pharmacometric analyses reported by Salazar et al. [14], the increased edoxaban exposure associated with amiodarone did not result in significant bleeding based on these model predictions and, therefore, no dose adjustment was recommended.
The coadministration of these 6 cardiovascular drugs with edoxaban was considered safe and well tolerated within our study. There did not appear to be any significant increases in TEAEs recorded during any coadministration, and rates of TEAEs considered to be related to any study drug were low. However, the studies assessed relatively small numbers of healthy subjects and excluded those who had recently received prescribed or over-the-counter systemic or topical medications or herbal supplements. The total observed bleeding events in these studies were relatively minor in number and mild in severity; therefore, they offer limited insight into the bleeding potential across these dose regimens. With these caveats in mind, the incidence of bleeding-related AEs did not portend any increased bleeding when edoxaban was coadministered with one of these specific cardiovascular medications, nor was there any significant trend for increases in other AEs. However, patients with AF tend to be elderly and have cardiovascular comorbidities that may require treatment with one or more additional medications. The AE profile for edoxaban coadministered with these cardiovascular drugs will be further evaluated in patients with AF or venous thromboembolism in the large phase 3 ENGAGE AF-TIMI 48 [5] and Hokusai-VTE [4] studies, respectively.
Total exposure of edoxaban with concomitant administration of quinidine, verapamil, or dronedarone increased <2-fold over the total exposure to edoxaban administered alone. While the pharmacometric analyses of the interactions between edoxaban and these 3 drugs suggest the potential for increased bleeding risk [14], the magnitude of these P-gp inhibitory effects was less than that which can be observed with drug interactions involving other mechanisms such as drug metabolizing enzymes. For example, statins that are extensively metabolized by CYP3A have shown a 10-fold or more increase in blood levels when coadministered with strong CYP3A inhibitors [12]. Therefore, the clinical relevance of the increases in exposure to edoxaban when coadministered with quinidine, verapamil, or dronedarone should be considered in the context of the therapeutic index for edoxaban, particularly for bleeding potential. The final determination of the relative effect of increased exposure on bleeding risk for edoxaban administered concomitantly with verapamil, quinidine, or dronedarone will be determined in the ENGAGE AF-TIMI 48 and Hokusai-VTE trials.
Cardiologists should be aware of emerging data characterizing the effect of the P-gp transport system on drug exposure and its potential for drug-drug interactions, especially for cardiovascular drugs with narrow therapeutic indices. While the potential for drug-drug interactions attributed to drug metabolizing enzymes are relatively well known, the results of these studies underscore the importance of drug-drug interactions involving P-gp. Potential drug-drug interactions involving transporters were the focus of a white paper published by the International Transporter Consortium in 2010 [9] and also the recent release of the FDA draft guidance on drug-drug interactions [12]. The FDA guidance states that all investigational drugs should be assessed using in vitro studies to determine the relative affinity of the compound for the P-gp transporter. Transporter assays measure the flux across various cell lines overexpressing P-gp (eg, Caco-2 and MDRK), and the relative flux (i.e., >2) can be used to define whether a drug is a P-gp substrate. The effect of P-gp on the disposition of various compounds has also been assessed in P-gp knockout mice relative to wild-type mice [27]. The FDA guidance further states that P-gp substrates characterized by in vitro assays as having significant potential for drug-drug interactions should be investigated in clinical studies. Dose administration guidance involving transporters should continue to expand for newly approved drugs. The role of the P-gp transporter system in various cardiovascular drugs is the focus of a very recent article in the Journal of the American College of Cardiology [28] and highlights the importance of understanding P-gp interactions that may occur between commonly co-prescribed drugs.
Our results suggest that quinidine, verapamil, and dronedarone—cardiovascular drugs used to manage AF patients that are likely to be prescribed with edoxaban—present some potential risk of bleeding in these patients if edoxaban is not dose-adjusted. Other recently developed oral anticoagulants (dabigatran, rivaroxaban, and apixaban) are also P-gp substrates [29–31]. Currently, in patients with normal renal function, no dose adjustments are recommended for patients who are also taking a P-gp inhibitor along with dabigatran or rivaroxaban [29, 30]. A dose adjustment is recommended for patients with moderate renal impairment who are receiving dabigatran and ketoconazole or dronedarone. Prescribing information for both rivaroxaban and apixaban, which are both metabolized by CYP3A4 and are P-gp substrates, indicate significant interactions with ketoconazole, ritonavir, clarithromycin, and erythromycin [30, 31]. Concomitant use of rivoraxaban with drugs that are both P-gp inhibitors and strong CYP3A4 inhibitors is to be avoided [30]. Patients receiving apixaban should have their dose reduced when administered along with a dual P-gp and strong CYP3A4 inhibitor or avoid their concomitant use altogether [31].
In conclusion, the results from this study indicate that the PK of edoxaban in healthy subjects is increased by coadministration of the potent P-gp inhibitors quinidine, dronedarone, and verapamil. Concomitant administration of each of these drugs with edoxaban significantly increased exposure to edoxaban as reflected by either Cmax, AUC, or 24-h concentrations relative to bleeding potential predicted from modeling and simulation of exposure-response relationships in patients with AF [14]. Atorvastatin, digoxin, and amiodarone did not significantly affect exposure to edoxaban. Coadministration of each tested drug with edoxaban appeared to be well tolerated in these short-term studies. The potential effects of coadministered P-gp inhibitors on the clinical efficacy and safety of edoxaban will be further evaluated in the large-scale phase 3 Hokusai-VTE and ENGAGE AF-TIMI 48 studies.
References
Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011;62:41–57.
Samama MM, Mendell J, Guinet C, Le Flem L, Kunitada S. In vitro study of the anticoagulant effects of edoxaban and its effect on thrombin generation in comparison to fondaparinux. Thromb Res. 2012;129(4):e77–82.
Lixiana (package insert). Tokyo, Japan: Daiichi Sankyo Co. Ltd.; 2011.
Raskob G, Buller H, Prins M, Segers A, Shi M, Schwocho L, van Kranen R, Mercuri M. The Hokusai VTEi. Edoxaban for the long-term treatment of venous thromboembolism: rationale and design of the Hokusai-VTE study. J Thromb Haemost. 2013. doi:10.1111/jth.12230
Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the effective aNticoaGulation with factor xA next GEneration in atrial fibrillation-thrombolysis in myocardial infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J. 2010;160(4):635–41.
Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, et al. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost. 2010;104(3):633–41.
Ogata K, Mendell-Harary J, Tachibana M, Masumoto H, Oguma T, Kojima M, et al. Clinical safety, tolerability, pharmacokinetics, and pharmacodynamics of the novel factor Xa inhibitor edoxaban in healthy volunteers. J Clin Pharmacol. 2010;50(7):743–53.
Bathala MS, Masumoto H, Oguma T, He L, Lowrie C, Mendell J. Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans. Drug Metab Dispos. 2012;40(12):2250–5.
International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–36.
Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36(2–3):179–94.
Marchetti S, Mazzanti R, Beijnen JH, Schellens JH. Concise review: clinical relevance of drug drug and herb drug interactions mediated by the ABC transporter ABCB1 (MDR1, P-glycoprotein). Oncologist. 2007;12(8):927–41.
US Food and Drug Administration. Guidance for industry. Drug interaction studies—design, data analysis, implications for dosing, and labeling instructions. 2012. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf. Accessed 20 May 2012.
Mikkaichi T, Yoshigae Y, Masumoto H, Imaoka T, Rozehnal V, Fischer T et al. Edoxaban transport via P-glycoprotein is a key factor for the drug disposition. Presented at the 18th North American Regional ISSX Meeting. 14–18 Oct 2012. Available at: http://c.ymcdn.com/sites/issx.site-ym.com/resource/resmgr/18th_NA_Meeting/Dallas_Online_Abstracts_Book.pdf. 2012 (Abstract P62).
Salazar DE, Mendell J, Kastrissios H, Green M, Carrothers TJ, Song S, et al. Modelling and simulation of edoxaban exposure and response relationships in patients with atrial fibrillation. Thromb Haemost. 2012;107(5):925–36.
Boyd RA, Stern RH, Stewart BH, Wu X, Reyner EL, Zegarac EA, et al. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J Clin Pharmacol. 2000;40(1):91–8.
Holtzman CW, Wiggins BS, Spinler SA. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26(11):1601–7.
Sziraki I, Erdo F, Beery E, Molnar PM, Fazakas C, Wilhelm I, et al. Quinidine as an ABCB1 probe for testing drug interactions at the blood–brain barrier: an in vitro in vivo correlation study. J Biomol Screen. 2011;16(8):886–94.
Multaq® (dronederone) tablets. Full prescribing information. Bridgewater, NJ: sanofi-aventis US, LLC; 2012.
Pacerone® (amioderone) 400 mg tablets. Full prescribing information. Minneapolis, MN: Upsher-Smith Laboratories, Inc.; 2010.
Mendell J, Noveck RJ, Shi M. Pharmacokinetics of the direct factor Xa inhibitor edoxaban and digoxin administered alone and in combination. J Cardiovasc Pharmacol. 2012;60(4):335–41.
Mendell J, Basavapathruni R, Swearingen D, Draves A, Zhang G, Morganroth J. A thorough electrocardiogram study of edoxaban, a novel factor Xa inhibitor. J Clin Pharmacol. 2011;51(8):1241–6.
Mendell J, Tachibana M, Shi M, Kunitada S. Effects of food on the pharmacokinetics of edoxaban, an oral direct factor Xa inhibitor, in healthy volunteers. J Clin Pharmacol. 2011;51(5):687–94.
Zahir H, Matsushima N, Halim AB, He L, Zhang G, Lee F, et al. Edoxaban administration following enoxaparin: a pharmacodynamic, pharmacokinetic, and tolerability assessment in human subjects. Thromb Haemost. 2012;108(1):166–75.
Rautio J, Humphreys JE, Webster LO, Balakrishnan A, Keogh JP, Kunta JR, et al. In vitro p-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab Dispos. 2006;34(5):786–92.
Weiss J, Haefeli WE. Evaluation of inhibitory potencies for compounds inhibiting P-glycoprotein but without maximum effects: f2 values. Drug Metab Dispos. 2006;34(2):203–7.
Wu X, Whitfield LR, Stewart BH. Atorvastatin transport in the Caco-2 cell model: contributions of P-glycoprotein and the proton-monocarboxylic acid co-transporter. Pharm Res. 2000;17(2):209–15.
Schinkel AH, Mol CA, Wagenaar E, van Deemter L, Smit JJ, Borst P. Multidrug resistance and the role of P-glycoprotein knockout mice. Eur J Cancer. 1995;31A(7–8):1295–8.
Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol. 2013; (in press).
PRADAXA® (dabigatran etexilate mesylate) Full prescribing information. Boehringer Ingelheim Pharmaceuticals Inc.; 2012.
XARELTO® (rivaroxaban) tablets. Full prescribing information. Titusville, New Jersey: Janssen Pharmaceutical, Inc.; 2011.
Eliquis® (apixaban) tablets for oral use. Full prescribing information. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
Acknowledgments
The authors would like to acknowledge editorial assistance provided by Evince Communications, Norwalk, CT, and by AlphaBioCom, LLC, King of Prussia, PA, which was funded by Daiichi Sankyo.
Role of Funding Source
This study was funded by Daiichi Sankyo Pharma Development, Edison, NJ.
Disclosures
Drs. Mendell, Zahir, Chen, Zhang, and Shi are employees of Daiichi Sankyo and also have stock options/long-term incentives in the company. Dr. Matsushima is an employee of Daiichi Sankyo Company Ltd. Dr. Noveck has served as principal investigator of multiple clinical trials, and has performed data reviews for Daiichi Sankyo, Eisai Inc., Chiesi Pharma, and Piramal Healthcare, all in therapeutic areas different from that of the present paper. Dr. Lee has nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mendell, J., Zahir, H., Matsushima, N. et al. Drug-Drug Interaction Studies of Cardiovascular Drugs Involving P-Glycoprotein, an Efflux Transporter, on the Pharmacokinetics of Edoxaban, an Oral Factor Xa Inhibitor. Am J Cardiovasc Drugs 13, 331–342 (2013). https://doi.org/10.1007/s40256-013-0029-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-013-0029-0