Abstract
Thrombosis is one of the most frequent complications affecting children with congenital heart disease (CHD). Palliative and reparative cardiac surgeries are some of the main players contributing to the thrombosis risk in this patient population. Additional risk factors related to the CHD itself (e.g., cardiac dysfunction, arrhythmias, and polycythemia in cyanotic cardiac disorders) can contribute to thrombogenicity alone or combined with other factors. Thrombotic complications have been recognized as a significant cause of morbidity and mortality in this patient population. Here, we provide an overview of the pathophysiology and risk factors for thrombosis as well as the indications for and use of different anticoagulation, antiplatelet, and thrombolytic agents. In addition, we describe some of most common thrombotic complications and their management in the pediatric CHD population.
Similar content being viewed by others
Thrombosis is one of the most frequent complications affecting children with congenital heart disease, leading to increased morbidity and mortality. |
There are multiple acquired and congenital risk factors that lead to this increased thrombotic risk. |
Monotherapy or combination therapy (anticoagulation with antiplatelets) and/or thrombolytic agents are used for different prophylactic or treatment indications in this patient population. |
Patients with single ventricle physiology are considered to be high risk for thrombosis complications and they require long-term thromboprophylaxis to decrease their thrombosis risk. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14754414
Introduction
Congenital heart disease (CHD) comprises one of the largest groups of congenital defects, affecting approximately 1% of births in the USA and Europe [1] and accounting for 4.2% of neonatal deaths in the USA [2]. Advances in critical care and surgical procedures have led to an increased survival of 83% in infants with CHD in the USA [3]. Despite this improvement in infant mortality, a recent meta-analysis demonstrated that survival gradually decreases over time, with a pooled 1-year survival of 87.0% [95% confidence interval (CI) 82.1–91.2], a pooled 5-year survival of 85.4% (95% CI 79.4–90.5), and a pooled 10-year survival of 81.4% (95% CI 73.8–87.9). Survival varies by CHD subtype, with the best survival in those with ventricular septal defects (96.3%; 95% CI 93.7–98.2) and lowest in those with hypoplastic left heart (12.5%; 95% CI 0.0–41.4) [1].
Thrombosis is a well-recognized life-threatening complication in children with CHD. Some CHD groups are considered to be at higher risk for thrombosis, such as single ventricles with shunts (shunt thrombosis 8–12% with 4% risk of death if shunt failure), central venous lines (CVL) (13% thrombosis complications), Fontan circulation (17–33%), arrhythmias, coronary aneurysms (Kawasaki disease), and cardiomyopathies (e.g., heart failure, myocarditis) [4]. A good understanding of the individual’s cardiac physiology and the associated thrombosis risk factors should provide the treating clinician with the knowledge to pursue preventive interventions for thrombosis, such as corrective surgery and/or pharmacological prophylaxis/therapy. In this article we review those thrombosis complications commonly encountered in CHD and provide an overview of thrombosis risk factors and the indications for use of pharmacologic therapy/prophylaxis based on previously conducted studies in pediatric CHD.
This article is based on previously conducted studies and does not contain any new studies with 80 human participants or animals performed by any of the authors.
Pathophysiology and Thrombosis Risk Factors in Children with CHD
Thrombosis is increasingly being recognized as one of the most frequent complications seen in children with CHD, particularly in hospitalized and critically ill children [5,6,7,8,9]. In a retrospective study by Manlhiot et al., the incidence of symptomatic thrombosis in children post-cardiac surgery was reported as 7.9%, compared to the 0.34–0.58% incidence of venous thromboembolic events reported by Raffini et al. [10]. In a retrospective study review of patients aged < 18 years within the Pediatric Health Information System (PHIS) database who underwent cardiac surgery from 2004 to 2012, thrombosis and mortality rates were compared with the demographic factors associated with each cardiac procedure. Of the 91,909 CHD patients who in the PHIS database who underwent surgery, 2655 (2.9%) developed thrombosis within the ensuing 12 months. The rate of thrombosis increased over time by 253% (p < 0.001), from 1.7% in 2004 to 4.4% in 2012. Systemic to pulmonary shunt placements (34.3%) and septostomy procedures (26.1%) had the highest thrombosis percentages. Children aged < 28 days had the highest thrombosis prevalence (61%). Those with thromboses had longer lengths of stay in the hospital and intensive care unit than those without (median stay in hospital 27 vs. 6 days; mean stay in intensive care unit 10 vs. 2 days, respectively; p < 0.001). In addition, the mean risk-adjusted cost was higher in those with thrombosis: US$126,257 versus $40,773 (p < 0.001). Thrombosis was also associated with higher rates of bacteremia (8.3 vs. 3.4%; p < 0.001), endocarditis (0.7 vs. 0.2%; p < 0.001), and mortality (12.3 vs. 0.8%; p < 0.001) [11].
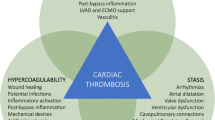
Multiple factors have been implicated in the pathophysiology of thrombosis in patients with CHD. Turbulent blood flow and areas of flow stasis are well documented in many patients with CHD. Blood flow disturbances, limited inflow/outflow, dilated atria or ventricles, and presence of potential thrombogenic foreign materials, such as sutures and stents/shunts, create an environment conducive to the activation of coagulation and thrombin generation [10] (Fig. 1). Utilization of cardiopulmonary bypass (CPB) is known to have bleeding and thrombogenic potential. CPB often leads to the disruption of blood flow and coagulation activation in the circuit. A procoagulant state in CPB can also develop due to platelet activation and inflammation, leading to thrombin generation. Children with CHD are at higher risk for coagulation derangement during CPB compared to adults due to their immature coagulation system, hemodilution of coagulation factors, hyperreactive platelets, physiologic changes associated with age (e.g., decreased antithrombin levels in infants) and/or due to polycythemia secondary to cyanosis (e.g., loss of plasma volume and hyperviscosity) [12, 13]. The degree of plasmatic anticoagulant and procoagulant derangements can lead to bleeding or thrombosis.
Pathophysiology of thrombosis in congenital heart disease (CHD). Different risk factors are implicated in thrombosis in patients with CHD, including Blood flow disturbances, limited inflow/outflow, dilated atria or ventricles, and presence of potential thrombogenic foreign materials, such as sutures and stents or shunts, utilization of cardiopulmonary bypass (CPB) during cardiac surgeries, use of central venous catheter (CVC) and patients with inherited thrombophilia. (Created with BioRender.com.)
Additional acquired thrombosis risk factors include the use of central venous catheters (CVC) or arterial catheters. The presence of an indwelling venous access line is generally considered to be one of the most important risk factors for thrombosis in pediatric patients, with nearly 20% of patients with CVC developing thrombosis [14]. Some studies have reported that certain palliative or corrective surgical procedures might be associated with a higher risk of thrombosis than others, such as correction of Tetralogy of Fallot (odds ratio 3.4) [15] and the Fontan procedure (thrombosis frequency 9%) [16]. Congenital thrombophilia, such as Factor V Leiden and Prothrombin 20210G-A mutations, may play a role in CHD thrombosis risk and have been reported with a frequency ranging from 6.3 to 38% among children with CHD who had experienced thrombotic complications [9, 17, 18].
Anticoagulant, Antiplatelet Agents and the Use of Thrombolysis in CHD
Three categories of medications are used in the therapy and/or prevention of thrombosis in pediatric patients with CHD: (1) anticoagulants, (2) antiplatelet agents, and (3) thrombolytic agents. A novel category of anticoagulants known as direct oral anticoagulants (DOACs) has been approved for use in adults with selected cardiac conditions, and approval for DOAC use in selected pediatric populations is pending completion of clinical trials and regulatory review/approval by the U.S. Food and Drug Administration (FDA) [4]. In this section we discuss the use of anticoagulants and antiplatelet agents in CHD; thrombolytic drugs will be discussed in the following section. The indications for the use, pharmacology, and dosing of each agent, anticoagulation monitoring, and its thromboprophylaxis role in CHD are summarized in Tables 1 and 2.
Anticoagulants
The class of anticoagulants includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), the pentasaccharide fondaparinux, warfarin, direct thrombin inhibitors (DTIs) and DOACs (Fig. 2).
Simplified diagrammatic representation of the coagulation pathway and the main target for the different anticoagulant medications. Unfractionated heparin and low-molecular-weight heparin (e.g., Enoxaparin) exert their anticoagulant effects primarily by binding to antithrombin, thus promoting inactivation of coagulation factors [mainly factors IIa (thrombin)] and Xa]. Warfarin inhibits the vitamin K-dependent synthesis of biologically active forms of clotting factors II, VII, IX, and X, as well as the regulatory factors protein C and protein S (not shown in this figure). Direct oral anticoagulants are either direct Xa inhibitors (rivaroxaban, apixaban, and edoxaban) or direct thrombin (IIa) inhibitors (dabigatran). The inverted red T reflects the mechanism of action or inhibition of the different anticoagulants. The blue arrows illustrate a simplified version of the coagulation cascade and its activation pathways
Unfractionated Heparin
This drug is composed of a heterogeneous mixture of highly sulfated glycosaminoglycans [19]. UFH exerts its anticoagulant effect by binding to the protease inhibitor antithrombin, thus promoting inactivation of coagulant factors IIa (thrombin), Xa, XIa, and XIIa (Fig. 2) [20, 21]. Heparin works via binding to antithrombin (AT); thus an AT deficiency leads to true physiological resistance (Table 1). To achieve steady state more quickly, intravenous (IV) UFH is usually started as a bolus followed by a continuous infusion [22]. The bolus should be deferred if there is a concern for significant bleeding, such as in neonates with a corrected gestational age of < 44 weeks and children with stroke who are at increased risk of intracranial bleeding. The anticoagulant activity of heparin is monitored using the partial thromboplastin time (aPTT) and/or anti-factor Xa activity (anti-FXa) levels. Several studies have demonstrated anti-FXa to be the superior assay to aPTT for monitoring heparin anticoagulation [23,24,25]. The aPTT can be prolonged in small children, especially in those who are critically ill, making aPTT a less reliable marker to monitor the heparin anticoagulant effect [23,24,25]. Pediatric patients represent a challenge for heparin therapy secondary to developmental hemostasis, with age-dependent changes in the target proteins for UFH, such as antithrombin [26]. Neonates and small children can present with heparin resistance due to physiologically low antithrombin levels. In addition, the clearance of UFH in infants is faster secondary to a larger plasma volume of distribution. For these reasons, this patient population usually requires higher doses of heparin than older children and adults to achieve a therapeutic anticoagulant effect [27] (Table 1).
The benefits of UFH include a short half-life and the ability to completely reverse the anticoagulation effect. Full reversal of heparin can be obtained with the use of protamine sulfate [28]. Approximately 1 mg of protamine will neutralize 100 U of UFH. Calculations should be made based on the total amount of heparin received in the prior 2–2.5 h [28]. In addition, UFH can be used in the setting of renal failure, whereas LMWH is relatively contraindicated. In clinically unstable patients at risk of bleeding who require anticoagulation, UFH should be strongly considered. Transition to an alternative anticoagulant should occur once the patient is clinically stable [4]. To minimize bleeding risk, concurrent antiplatelet therapy should be avoided if possible. Non-hemorrhagic complications associated with UFH include heparin-induced thrombocytopenia (HIT) and osteopenia [4]. Pediatric patients undergoing CPB are among the patients at higher risk of HIT [29]. The treatment of HIT includes discontinuation of heparin and avoidance of LMWH (e.g., enoxaparin). A DTI (i.e., argatroban, bivalirudin) or fondaparinux is recommended when HIT is suspected and/or confirmed [4].
Low-Molecular-Weight Heparin
Low-molecular-weight heparin is derived from UFH by depolymerization. The resulting changes lead to HMWH having clinical advantages over UFH and provide the LMWH with increased stability [30, 31]. Similar to UFH, LMWH exerts its anticoagulant effect by its AT-mediated selective inhibition of factor Xa (Fig. 2). However, when compared with UFH, there is a reduced inhibitory activity against factor IIa (thrombin) relative to factor Xa [4]. Clearance of LMWH depends on renal excretion, resulting in a half-life that is two- to fourfold longer than that of UFH. It is advised to use LMWH with caution when creatinine clearance is < 30 mL/min [32]. Enoxaparin is currently the most common LMWH used and studied in pediatric patients [33]. Pediatric patients exhibit a significant variation in LMWH dosing based on age and weight, with younger children and neonates requiring higher doses of LMWH secondary to increased volume of distribution, increased renal clearance, and developmental hemostasis [22, 34] (Table 1). Baseline laboratory values should be obtained prior to initiating LMWH therapy [complete blood count, prothrombin time (PT), aPTT, and serum creatinine] [4].
The anti-Xa assay specific for LMWH is used for anticoagulation monitoring. The dose is adjusted to a peak level of 0.5–1 U/mL (drawn 4–6 h post administration of the third or fourth dose). Clinicians are encouraged to refer to Monagle et al. [22] for adjustment recommendations based on peak anti-Xa level. LMWH can be partially reversed (approx. 70%) with protamine sulfate [4]. The most common risk associated with LMWH therapy is bleeding. LMWH can be used in clinically stable patients with minimal or no bleeding risk and good renal function. HIT has been reported with the use of LMWH in pediatric patients [35]. Overall, HIT induced by LMWH is thought to be less frequent when compared with UFH [36].
Fondaparinux
Fondaparinux is a synthetic preparation of the pentasaccharide sequence found in heparin manufactured to a high degree of purity and uniformity [32]. The antithrombotic activity of fondaparinux is the result of AT-mediated selective inhibition of factor Xa. By selectively binding to AT, fondaparinux potentiates (by about 300-fold) the neutralization of factor Xa by AT. Fondaparinux does not inactivate thrombin [32]. Fondaparinux is administered via a subcutaneous injection. The half-life is longer than LMWH (17 h) and its clearance is exclusively renal [4].
Fondaparinux pediatric dosing is 0.1 mg/kg subcutaneous daily [37] (Table 2). Specific assays calibrated for fondaparinux should be used for anti-Xa coagulation monitoring [38]. Similar to other anticoagulants, the highest risk associated with fondaparinux therapy is bleeding. Due to its long half-life and the lack of a reversal agent, this medication should only be used in patients who are clinically stable with a low risk of bleeding; fondaparinux should not be used in patients with renal insufficiency [4]. Off-label use has been reported in both adults and pediatric patients in the treatment of HIT [39, 40].
Warfarin
Warfarin inhibits the vitamin K-dependent synthesis of biologically active forms of clotting factors II, VII, IX, and X, as well as the regulatory factors protein C and protein S (Fig. 2). It has a long half-life (approx. 35 h) and therefore needs to be given only once daily. It takes several days for warfarin to achieve a therapeutic effect because it affects only newly synthesized coagulation factors, not circulating coagulation factors [32]. Warfarin is metabolized in the liver by human cytochrome P450 (CYP). One limitation to warfarin use is potential warfarin–drug interactions that can occur over a very wide range of drugs that are metabolized by these CYP isoforms; a large number of such interactions have been reported [32].
The interaction of age, diet, and varying therapeutic target ranges together with genetic polymorphism of enzymatic breakdown makes warfarin dosing and anticoagulation a challenge in the pediatric population [4]. Warfarin recipients attain an international normalized ratio (INR) of > 2 and most reach a target INR in < 7 days [22, 41]. Warfarin monitoring relies on the INR that is calculated based on the PT test result. INR values are used to standardize results across different laboratories; therapeutic ranges vary with the indication for anticoagulation. Dosing on subsequent days is based on the daily INR [22] (Table1). Patients who have had a Fontan procedure, especially those who have underlying liver disease, are generally more sensitive to the effects of warfarin, and so a conservative loading dose of 0.1 mg/kg is recommended. Patients aged < 1 year often require much higher doses of warfarin [4]. Of note, bridging therapy with heparin is always recommended upon the initiation of warfarin and until the therapeutic INR is achieved. Bridging with heparin is also recommended in those patients on chronic warfarin therapy prior to invasive procedures regardless of the presence or absence of thrombus. Reversal of warfarin is related to the urgency in which reversal is desired, the thrombotic risk associated with the treatment, and the severity of bleeding [42, 43]. Choices for reversal include vitamin K, fresh frozen plasma, and discontinuation of warfarin. Agents approved for anticoagulation reversal in life-threatening bleeding, such as 4-factor-prothrombin factor concentrates [4F-PCC] (e.g., Kcentra®), however, are not approved for use in pediatric patients and, therefore, dosing recommendations are not available [44]. The use of a particular agent to reverse warfarin anticoagulation in the setting of acute or life-threatening bleeding needs to take into consideration the particular thromboembolic risk in each patient as some cardiac conditions, such as left ventricular assist devices and mechanical prosthetic valves portend a higher thrombosis risk [4]. In terms of CHD, warfarin is most often used in patients who have undergone heart valve(s) replacement. Ranges for therapeutic INR depends of the type of heart valve (e.g., brand), anatomic location (e.g., aortic and/or mitral vs. tricuspid) in addition to underlying cardiac function (Tables 1, 2) [4].
Parenteral Direct Thrombin Inhibitors
Direct thrombin inhibitors are direct, specific, and reversible inhibitors of thrombin. The three commercially available DTIs are bivalirudin, argatroban, and lepirudin [4]. DTIs are administered intravenously. The elimination half-life of argatroban is 45 min and it is principally excreted by the biliary route. Lepirudin is a recombinant hirudin with a half-life of 60 min, which is cleared by the kidneys. Bivalirudin has a half-life of 25 min and is cleared by the liver and kidneys (Table 1) [45]. DTI dosing recommendations for pediatric patients are extrapolated from adult experience/protocols (Table 1). In patients with advanced liver disease, argatroban dosing must be reduced [46]. Most institutions report titrating the DTIs based on aPTTs every 2–4 h to aim for an aPTT of 1.5–2.5 × normal [47, 48]. The labeled indications for DTIs are HIT and percutaneous coronary intervention. At present, these agents are not FDA approved for use in children; however their use in HIT is accepted and the pediatric experience with its use continues to grow [4, 49, 50]. Hemorrhagic complications, particularly when combined with thrombolytic or antiplatelet agents, can be life threatening. There is no antidote to any of these agents, so the antithrombotic effect cannot be reversed pharmacologically [32].
Direct Oral Anticoagulants
Direct oral anticoagulants are currently approved for use in the adult population. There are two broad categories of DOACs: direct anti-Xa inhibitors (rivaroxaban, apixaban, and edoxaban) and direct thrombin (IIa) inhibitors (dabigatran) (Fig. 1) [4]. None of these drugs have FDA approval for use in children. Some pediatric DOAC trials have been completed while others are ongoing, particularly in patients with CHD [51,52,53]. The advantages of DOACs include more predictable pharmacokinetics, a wide therapeutic window, and limited drug–drug interactions when compared to warfarin. They also have a relatively rapid onset of action and shorter half-life than warfarin [4]. The EINSTEIN Junior phase II and III clinical trials validated the predicted body weight-adjusted rivaroxaban regimens reported from the phase 1 study in children aged < 18 years and also reported similar safety and efficacy of rivaroxaban in pediatric patients with venous thromboembolism when compared to standard anticoagulants [54, 55]. However, there is currently insufficient data on the use of rivaroxaban in pediatric patients with CHD. There is, however, an ongoing clinical trial aimed at assessing the safety of DOACs in pediatric cardiac patients [52]. A clinical trial comparing dabigatran with warfarin in adult patients with either an aortic or mitral mechanical heart valve was halted early secondary to increased thrombotic and bleeding events in patients receiving dabigatran in comparison to those receiving warfarin [53]. At this time, DOACs should not be used in the setting of mechanical heart valves.
Antiplatelet Agents
Aspirin-Acetylsalicylic Acid
Aspirin irreversibly inhibits cyclooxygenase (COX-1 and COX-2) activity in the arachidonic acid pathway, inhibiting the formation of thromboxane (TXA2), resulting in inhibition of platelet activation and aggregation. Aspirin is hepatically metabolized and renally excreted. Although dose dependent, the half-life (t1/2) is 15–20 min [56] (Table 1); however platelet inhibition lasts for the life span of the platelets. Larger doses of ASA can have a longer half-life. There are no reversal agents for those patients receiving aspirin. If bleeding is encountered, it is recommended to discontinue the drug and provide platelet transfusion if bleeding is not well controlled. Full platelet function usually returns after 4 days of aspirin discontinuation; however due to irreversible platelet inhibition, it is commonly recommended to discontinue ASA 7 days before the surgery to allow full platelet regeneration and function [4].
Low-dose aspirin has become the mainstay of thromboprophylaxis for Blalock–Taussig shunts [22, 57]. Aspirin is often used as a part of the thromboprophylaxis regimen with VADs, although the specifics vary with the type of device. Opinions vary on the use of aspirin in patients with stage-I palliation of hypoplastic left heart syndrome via the Sano modification, patients with single ventricle physiology beyond stage I in general, and patients with intravascular stents [22, 57]. The use of aspirin is recommended for non-embolic stroke (please refer to section Stroke for management) (Tables 1, 2) [22, 57, 58].
Clopidogrel
Clopidogrel undergoes hepatic transformation prior to irreversibly blocking the P2Y12 component of ADP receptors, inhibiting platelet aggregation, thus blocking activation of the glycoprotein (GP)IIb/IIIa pathway [32]. It is a prodrug, so its antiplatelet effects can be seen for up to 14 days after the last dose due to conversion of the pro-drug to the active drug. No reversal agents are available [32]. Dosing varies with age in infants and children (Table 1). Clopidogrel is used instead of ASA in the presence of ASA allergy or intolerance [4].
Other agents, such as ticagrelor and prasugrel, bind to P2Y12 receptors reversibly and irreversibly, respectively. Both drugs have a more potent, rapid onset of action, and predictable platelet inhibition when compared to clopidogrel [59, 60]. These agents are only approved in adults with coronary syndromes at this time.
Dipyridamole
Dipyridamole inhibits the activity of adenosine deaminase and phosphodiesterase, causing an accumulation of adenosine, adenine nucleotides, and cyclic AMP which in turn inhibits platelet aggregation [4, 57]. Intravenous and oral formulations are available (Table 1). Currently, the main use of dipyridamole is as adjunct therapy to other anticoagulant/antiplatelet medications.
Thrombolysis-Tissue Plasminogen Activator
In contrast to anticoagulants that decrease the body’s ability to form new thrombus, thrombolytic agents act by converting plasminogen to plasmin and thereby actively reducing the clot burden [61]. Commonly used thrombolytic agents are (recombinant DNA origin) forms of the enzyme human tissue-type plasminogen activators (tPA) alteplase (tPA), reteplase and tenecteplase [4]. Alteplase is most commonly used in pediatrics due to its short half-life of 3–5 min [61]. Thrombolytic therapy can be given as catheter-directed or systemic IV infusion. Numerous dosing strategies for tPA have been used in the pediatric population, and there is currently no consensus as to which approach is optimal. Systemic therapy can be given as high-dose or low-dose therapy [4, 61] (Table 1). Thrombolytic therapy in children should be restricted to situations in which the benefit of rapid thrombus resolution is thought to outweigh the risk of major hemorrhage and is usually reserved for thrombosis which is causing hemodynamic compromise [e.g., pulmonary embolism (PE) with cardiac dysfunction, mechanical valve thrombosis, stent occlusion leading to poor cardiac function or oxygenation] and/or arterial thrombosis causing poor perfusion and tissue ischemia [61].
Thrombosis complications and their management
Stroke
Approximately 20% of all strokes among children are secondary to CHD. In addition, cardiac surgery in itself contributes to stroke risk [62, 63]. A recently published American Heart Association (AHA) scientific statement reviewed diagnostic evaluation and stroke management in infants and children [58]. This scientific statement provides a comprehensive, evidence-based guideline for the management of ischemic stroke, intracranial bleeding, and cerebral venous sinus thrombosis (CVST) in CHD. A few major points are highlighted below.
Patients with complex CHD with right-to-left shunting and cyanosis are particularly prone to strokes; however, other types of cardiac defects can predispose patients to neurological events. CHD is also a risk factor for CVST [58]. Stroke is more prevalent among children with uncorrected CHD [64]. Emboli can arise at the atrial level (e.g., atrial septal defect with pulmonary hypertension), at the ventricular level (e.g., ventricular septal defect with pulmonary hypertension), or at the arterial level (e.g., pulmonary arteriovenous fistula) [58]. Paradoxical emboli result when thrombi bypass the pulmonary circulation during right-to-left shunting; they also occur in those individuals with pulmonary arteriovenous fistula, typically seen in hereditary hemorrhagic telangiectasia (Osler–Weber–Rendu disease) [58]. Thromboembolic stroke can also occur after cardiac catheterization and major cardiac surgery. During cardiac surgery, systemic hypoperfusion or hypoxia and reperfusion during or following CPB can potentially result in stroke. Reperfusion of the brain leads to oxygenation and the generation of free radicals, especially reactive oxygen species (ROS) and reactive nitrous species (RNS), which have intense oxidation or nitrification potentials in the human brain. During cerebral ischemia reperfusion, especially with blood reflow, massive generation of ROS and RNS leads to cell death via DNA damage, protein dysfunction, and lipid peroxidization [65].
Surgical repair of CHD reduces but does not always eliminate the long-term risk of thromboembolism [66]. Ischemic strokes in the setting of CHD may be classified as cardioembolic (with a defined source) or cryptogenic (with no definite source) [57]. When evaluating a child with CHD who has developed a stroke, it is important to identify underlying risk factors, such as intracardiac emboli, by performing transthoracic echocardiography to identify whether any other cardiac risk factors for thrombi, such as chamber dilation or ventricular dysfunction, are present. Transesophageal echocardiography may be necessary if transthoracic imaging is deemed suboptimal or non-diagnostic. Magnetic resonance imaging (MRI) is the most sensitive and accurate tool for detecting stroke and cerebral venous sinus thrombosis (CVST) [67, 68]. Additional potential etiologies for stroke, such vertebral artery dissections and underlying hypercoagulability, should also be considered if other evaluations have been non-diagnostic [58].
In patients with CHD, it is important to identify those patients at significant risk for stroke. According to the AHA stroke committee, when feasible, complex heart defects with a high stroke risk should be repaired both to improve cardiac function and to reduce the risk for stroke. Therapy for congestive heart failure may reduce the likelihood of cardiogenic embolism [58]. Recommendations are to initiate anticoagulation in those children with suspected cardioembolic stroke [57]. Duration of anticoagulation should be individualized based on the status of cardiac defect(s) and/or function and the risk for stroke recurrence [57, 58]. Transition to aspirin is reasonable in children judged to have a low risk for cardioembolic stroke. There are insufficient data to support a recommendation concerning the risk versus benefit of antithrombotic treatment for stroke that is judged to be related to patent foramen ovale (PFO) [57, 58]. Mounting evidence suggests that percutaneous devuce closure of the PFO is more effective than antiplatelet therapy alone for reducing the risk of recurrent stroke in select adult patients with an embolic-appearing ischemic stroke who have a PFO with a right-to-left interatrial shunt and who have no other identified cause or mechanism of stroke. The risk of recurrent stroke with device closure is reduced by approximately 60% compared with medical therapy (e.g., from about 5% to 2% during a 3- to 6-year period). For patients with an embolic-appearing ischemic stroke who have a medium- to high-risk PFO and no other evident source of stroke despite a comprehensive evaluation and who have a concurrent indication for cardiac surgery (e.g., indication for valve surgery), surgical closure of PFO for secondary stroke prevention after PFO-associated stroke is appropriate [69].
The left atrial appendage is an anatomical region of the left atrium with different anatomical shapes that are believed to be the source of emboli primarily in patients with atrial fibrillation. Left atrial appendage occlusion (LAAO) is a possibility in adult patients with atrial fibrillation at high risk of bleeding or relative/absolute contraindication for long-term anticoagulation (Class IIbB) or in those with atrial fibrillation undergoing cardiac surgery (Class IIbC) [70]. In other conditions with very high thromboembolic risk, such as patients with mechanical prostheses, anticoagulation cannot be stopped despite LAAO, although it could be considered as a means to reduce embolic risk. The European Heart Rhythm Association/European Association of Percutaneous Cardiovascular Interventions expert consensus statement on catheter-based LAA occlusion reached different conclusions with respect to the indications and anticoagulation regimen for LAA closure. These associations introduced an algorithm that is designed to identify patients who will benefit most from LAA interventional closure and patients without a contraindication for an anticoagulation regimen [71]. The benefits of left atrial occlusion in the pediatric population, however, is yet to be determined.
Vascular Thrombosis After Cardiac Catheterization
The incidence of vascular thrombosis post-cardiac catheterization varies significantly in the literature, ranging from 0 to 32% [72,73,74,75,76,77]. However, it is been reported that vascular (venous and arterial) thrombosis is frequently encountered after cardiac catheterization and that this risk can be reduced by procedural anticoagulation [72]. It is recommended to initiate procedural anticoagulation with 100 U/kg bolus of UFH and to monitor anticoagulation effects with activating clotting times (ACT) 1 h after heparin bolus and every 30 min thereafter. Intravenous heparin infusion should be resumed immediately after bolus infusion at 50–100 U/kg per hour and adjusted accordingly to maintain an ACT > 200 s. Prophylaxis with ASA alone is not recommended.
Despite the generalized use of UFH for thromboprophylaxis during cardiac catheterization, femoral artery thrombosis remains a frequent complication, usually presenting as diminished or absent pulse or other signs and symptoms of limb ischemia. The recommended treatment for patients with lower-extremity arterial pulse loss and/or evidence of limb ischemia after cardiac catheterization is UFH. If limb ischemia persists despite adequate heparinization, surgical thrombectomy or thrombolysis should be considered and the choice made after assessment of the patient’s bleeding risk [57]. Pediatric antithrombotic guidelines recommend therapeutic doses of intravenous UFH as initial therapy for neonates and children with acute femoral artery thrombosis, with subsequent conversion to LMWH or else continuation of UFH for a total of 5–7 days [22]. For neonates and children with limb perfusion that fails to respond to initial UFH therapy and who have no known contraindications to tPA, thrombolysis is recommended. Surgical intervention is recommended for those with contraindication for thrombolysis in the setting of excessive bleeding risk, organ dysfunctionm, and/or progressive limb ischemia [22].
Deep vein thrombosis (DVT) is also a frequently encountered complication following cardiac catheterization. Some of these events are often subclinical and diagnosed when vascular access is not possible at the time of subsequent cardiac catheterization or CVC placement [78]. Symptomatic DVTs usually present with swelling, pain, or color changes of the affected extremity. Recommendations for anticoagulation are the same for children with or without CHD who present with DVTs. Anticoagulation with LMWH or warfarin is recommended for a minimum of 3 months in those patients with provoked DVT [79].
CVL-Associated Thrombosis
Children with CHD undergoing cardiac surgery often require the placement of a central venous and/or arterial line. Endothelial damage after central venous or arterial access is often the underlying precipitating factor for thrombosis [80]. Anticoagulation management for catheter-associated arterial and venous thrombosis is no different from thrombosis management following cardiac catheterization. If a DVT is associated with a CVC and the CVC is still required for patient care, the recommendations are to initiate anticoagulation rather to remove the CVC, as catheter insertion at another venous site will place the patient at risk for another venous thrombosis [79]. However, if the CVC is non-functional or not needed any longer, removal is recommended. Initiation of anticoagulation is suggested for 3–5 days prior to catheter removal [79]. For neonates and children with a peripheral arterial catheter-related thromboembolism, immediate removal of the catheter is recommended [22].
Pulmonary Embolism
Pulmonary artery thrombosis can result from in situ thrombosis or embolism from an anatomically distant site. The true incidence of PE in children is unknown. However, the incidence of PE in children with documented DVT has been reported to range from 30 to 60%, which is similar to that of adults with DVT [81, 82]. PE can present as acute chest pain, shortness of breath and, on rare occasions, hemoptysis. Large pulmonary arterial thromboses (e.g., massive) can result in hypotension, cardiac dysfunction, hemodynamic instability, and death.
The American Society of Hematology (ASH) pediatric guidelines recommend anticoagulation with LMWH or warfarin for at least 3 months, in those children with provoked PE regardless of the degree of severity (e.g., massive, sub-massive, low risk). Thrombolysis (catheter directed or systemic) is recommended in those patients at low or no risk for bleeding who present with hemodynamic instability. The use of inferior vena cava filters is not recommended [79]. However, the AHA committee suggest vena cava filter placement in children with heart disease and a PE with a contraindication to anticoagulation (e.g., active or high risk for hemorrhage) or those who failed anticoagulation and have progressive PE [57].
Intracardiac Thrombosis
Intracardiac thromboses are most commonly frequently diagnosed in children who have a CVC extending into the right atrium and in those patients with dilated cardiomyopathy [83, 84]. Intracardiac thrombosis should be treated with systemic anticoagulation for at least 3 months [83]. Patients with intracardiac thrombosis causing hemodynamic instability or those at high risk for embolization (e.g., mobile thrombus located in an area of high flow) should receive thrombolytic therapy or surgical thrombectomy [57]. Antiplatelet therapy is recommended following device closure of an atrial or ventricular septal defect to help prevent intracardiac thrombosis (Table 2).
Stent Thrombosis
The use of stents in pediatric patients with CHD has expanded over time, especially for those with stenotic vascular lesions or patients that require ductus arteriosus patency to maintain either systemic or pulmonary blood flow [85, 86]. Procedural anticoagulation is recommended with IV UFH in children undergoing stent placement. For low-risk stents, post-operative thromboprophylaxis with ASA for at least 6 months is recommended. If the stent placement is considered to be high risk for thrombosis (e.g., non-pulsatile flow, previous complete occlusion, presence of a thrombophilic abnormality, etc.), it is reasonable to use warfarin or LMWH with or without antiplatelet therapy for 3–6 months after implantation and then continue or institute antiplatelet therapy alone [57].
Shunt Thrombosis
Acute shunt thrombosis has long been a recognized complication after pediatric cardiac surgery. The incidence of shunt thrombosis in infants has been reported to be 8–12% in different case series and is usually life-threatening unless quickly resolved [87, 88]. Patients requiring shunt intervention have been reported to have a significantly higher incidence of infection, higher need for extracorporeal membrane oxygenation (ECMO), and a longer hospital stay. Fenton et al. reported an overall 4% risk of death resulting from shunt thrombosis, with one third of patient deaths occurring between hospital discharge and the next planned cardiac surgery due to this complication [89]. In the absence of an increased risk of bleeding, long-term use of low-dose aspirin is recommended therapy for the prevention of long-term polytetrafluoroethylene systemic-pulmonary shunt thrombosis in infants and children [57]. In infants and children with a recently placed polytetrafluoroethylene systemic-pulmonary artery shunt who have an increased risk for thrombosis (e.g., suspected or confirmed infection, known CVC-associated thrombus, stented shunt, or hypercoagulable state), systemic heparinization is recommended in the early postoperative period [57]. In the event of clinical evidence of acute polytetrafluoroethylene systemic-pulmonary shunt thrombosis, immediate intervention should be initiated. Systemic anticoagulation with a bolus of intravenous heparin (50–100 U/kg) is suggested together with consideration of ongoing heparin infusion. Interventional catheterization, manual shunt manipulation, surgical shunt revision, or ECMO should be considered if thrombosis does not resolve [57].
Artificial Valve Thrombosis
Overall, the incidence of prosthetic valve thrombosis is reported to be between 0.1 and 5.7% per patient-year [90]. The incidence is 0.5–6% in the aortic and/or mitral positions and up to 20% in the tricuspid position [91]. Thrombolysis should be considered in those patients with low or minimal bleeding risk with thrombosis who had failed anticoagulation and/or demonstrated impaired blood flow [57, 92,93,94,95,96,97,98]. Guidelines from the American College of Cardiology/AHA, European Society of Cardiology, and the American College of Chest Physicians recommend anticoagulation after placement of an artificial valve, either surgically or percutaneously [99,100,101]. In general, low flow blood systems or the possibility of systemic embolization require more aggressive anticoagulation. As even newer valves continue to be made available for replacement purposes in the pediatric population, future studies will be required to determine the optimal anticoagulation regimen [102,103,104,105].
Special Group: Single Ventricle
Patients with single ventricle physiology are considered to be at high risk for thrombotic complications. The etiology of thrombotic complications in this population is multifactorial in nature but includes elements of blood hypercoagulability, resistance to anticoagulation, and blood flow disruption [106]. Most patients with single ventricle physiology are managed by a three-staged surgical pathway [107]. The highest thrombosis risk period is during the initial palliation. The incidence of Blalock–Taussig shunt thrombosis has been reported to be in the range of 1–17% [108, 109]. Recent studies have reported shunt thrombosis to be a major cause of shunt failure and mortality in these patients [89, 110]. A cross-sectional study reported a higher incidence of thrombotic complications of 40 and 28% after initial palliation and superior cavopulmonary connection, respectively [106]. In addition, patients undergoing the third surgical procedure (Fontan procedure) have documented coagulation and liver abnormalities prior to and after completion of the Fontan procedure [111,112,113,114]. A recent long-term follow-up study of patients having the Fontan procedure showed that 25% of late deaths were attributable to thrombotic complications [115]. In an adult Fontan population, 17% of patients were found to have silent pulmonary embolism 14 years after the Fontan procedure; in comparison, the prevalence was 24% in non-anticoagulated patients [116]. The risk of thrombotic complications is also dependent on the type of Fontan procedure performed as well as the presence of any atrial arrhythmias [117, 118]. Transthoracic echocardiography performed as part of the routine follow-up of Fontan patients remains the mainstay for thrombosis surveillance [57].
Table 2 summarizes the recommended thromboprophylaxis for procedures and underlying cardiac conditions in children [57] The effect of short-term and long-term thromboprophylaxis on the incidence of thrombotic complications has been the focus of many studies. Prophylaxis with warfarin or LMWH may be a reasonable therapeutic option in infants and children for 3–12 months after the Fontan procedure, especially if they have had previous thrombotic issues, while long-term antiplatelet therapy for the prevention of thrombosis is a reasonable option after the Fontan procedure. Long-term therapy with warfarin may be the reasonable therapeutic choice after the Fontan procedure for patients with anatomic or hemodynamic risk factors for thrombosis. Initiation of antithrombotic therapy or an increase in the magnitude of antithrombotic therapy (e.g., change in agent from antiplatelet to anticoagulant or higher anticoagulation target ranges) for prophylaxis after the Fontan procedure may be the reasonable choice for adolescent or adult patients (Table 2) [57]. That being said, recent studies have documented no increased risk of thrombosis events and a lower risk of adverse events in patients only on antiplatelet therapy versus those on patients on anticoagulation therapy [119,120,121]. The safety and efficacy of newer agents in this patient population, such as DOACs, is yet to be determined.
Conclusions
As survival continues to improve in patients with CHD, treatment measures to prevent long-term morbidity and mortality, especially those related to thrombotic complications, have become essential. As newer anticoagulation medications are brought to market, rigorous evaluation in patients with CHD will be needed to determine safety and efficacy. The need for multidisciplinary collaboration, especially between cardiology and hematology, is essential to optimize the care of this complex patient population in regards to hematological issues.
References
Best KE, Rankin J. Long-term survival of individuals born with congenital heart disease: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5:e002846.
Petrini JR, Broussard CS, Gilboa SM, Lee KA, Oster M, Honein MA. Racial differences by gestational age in neonatal deaths attributable to congenital heart defects—United States, 2003–2006. MMWR Morb Mortal Wkly Rep. 2010;59:1208–11.
Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131:e1502–8.
Giglia TM, Witmer C, Procaccini DE, Byrnes JW. Pediatric Cardiac Intensive Care Society 2014 consensus statement: pharmacotherapies in cardiac critical care anticoagulation and thrombolysis. Pediatr Crit Care Med. 2016;17:S77–88.
Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83:1251–7.
van Ommen CH, Heijboer H, Büller HR, Hirasing RA, Heijmans HSA, Peters M. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J Pediatr. 2001;139:676–81.
Monagle P, Adams M, Mahoney M, et al. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47:763–6.
Kim S-J, Sabharwal S. Risk factors for venous thromboembolism in hospitalized children and adolescents: A systemic review and pooled analysis. J Pediatr Orthop Part B. 2014;23:389–93.
Ören H, Devecioğlu Ö, Kemahli S, et al. Analysis of pediatric thrombotic patients in Turkey. Pediatr Hematol Oncol. 2004;21:573–83.
Manlhiot C, Menjak IB, Brandao LR, et al. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation. 2011;124:1511–9.
Silvey M, Hall M, Bilynsky E, Carpenter SL. Increasing rates of thrombosis in children with congenital heart disease undergoing cardiac surgery. Thromb Res. 2018;162:15–21.
Gruenwald CE, Manlhiot C, Crawford-Lean L, et al. Management and monitoring of anticoagulation for children undergoing cardiopulmonary bypass in cardiac surgery. J Extra Corpor Technol. 2010;42:9.
Monagle P, Barnes C, Ignjatovic V, et al. Developmental haemostasis. Impact for clinical haemostasis laboratories. Thromb Haemost. 2006;95:362–72.
Andrew M, Monagle PT, Brooker L. Thromboembolic complications during infancy and childhood. Shelton: PMPH USA; 2000.
Alioglu B, Avci Z, Tokel K, Atac FB, Ozbek N. Thrombosis in children with cardiac pathology: analysis of acquired and inherited risk factors. Blood Coagul Fibrinolysis. 2008;19:294–304.
Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg. 2001;71:1990–4.
Ehrenforth S, Junker R, Koch HG, et al. Multicentre evaluation of combined prothrombotic defects associated with thrombophilia in childhood. Eur J Pediatr. 1999;158:S97–104.
Ong BC, Zimmerman AA, Zappulla DC, Neufeld EJ, Burrows FA. Prevalence of factor V Leiden in a population of patients with congenital heart disease. Can J Anaesth. 1998;45:1176–80.
Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999(Suppl 3);25:5–16.
Damus PS, Hicks M, Rosenberg RD. Anticoagulant action of heparin. Nature. 1973;246:355–7.
Rosenberg JS, McKenna PW, Rosenberg RD. Inhibition of human factor IXa by human antithrombin. J Biol Chem. 1975;250:8883–8.
Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S–801S.
Liveris A, Bello RA, Friedmann P, et al. Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2014;15:e72–9.
Samuel S, Allison TA, Sharaf S, et al. Antifactor Xa levels vs. activated partial thromboplastin time for monitoring unfractionated heparin. A pilot study. J Clin Pharm Ther. 2016;41:499–502.
Chan AK, Black L, Ing C, Brandão LR, Williams S. Utility of aPTT in monitoring unfractionated heparin in children. Thromb Res. 2008;122:135–6.
Newall F, Johnston L, Ignjatovic V, Monagle P. Unfractionated heparin therapy in infants and children. Pediatrics. 2009;123(3):e510–8.
McDonald MM, Jacobson LJ, Hay WW Jr, Hathaway WE. Heparin clearance in the newborn. Pediatr Res. 1981;15:1015–8.
Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e24S–43S.
Risch L, Huber AR, Schmugge M. Diagnosis and treatment of heparin-induced thrombocytopenia in neonates and children. Thromb Res. 2006;118:123–35.
Hirsh J, Raschke R, Warkentin TE, Dalen JE, Deykin D, Poller L. Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1995;108:258s–75s.
Hirsh J, Levine MN. Low molecular weight heparin: laboratory properties and clinical evaluation. A review. Eur J Surg Suppl Acta Chir Suppl. 1994;571:9–22.
Royston D. 45—anticoagulant and antiplatelet therapy. In: Hemmings HC, Egan TD, editors. Pharmacology and physiology for anesthesia (second edition). Philadelphia: Elsevier; 2019. p. 870–94.
Dix D, Andrew M, Marzinotto V, et al. The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr. 2000;136:439–45.
Bauman ME, Belletrutti MJ, Bajzar L, et al. Evaluation of enoxaparin dosing requirements in infants and children. Better dosing to achieve therapeutic levels. Thromb Haemost. 2009;101:86–92.
Vakil NH, Kanaan AO, Donovan JL. Heparin-induced thrombocytopenia in the pediatric population: a review of current literature. J Pediatr Pharmacol Ther. 2012;17:12–30.
Warkentin TE, Levine MN, Hirsh J, et al. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. N Engl J Med. 1995;332:1330–5.
Young G, Yee DL, O'Brien SH, Khanna R, Barbour A, Nugent DJ. FondaKIDS: A prospective pharmacokinetic and safety study of fondaparinux in children between 1 and 18 years of age. Pediatr Blood Cancer. 2011;57:1049–54.
Paolucci F, Frasa H, Van Aarle F, et al. Two sensitive and rapid chromogenic assays of fondaparinux sodium (Arixtra) in human plasma and other biological matrices. Clin Lab. 2003;49:451–60.
Schindewolf M, Steindl J, Beyer-Westendorf J, et al. Frequent off-label use of fondaparinux in patients with suspected acute heparin-induced thrombocytopenia (HIT)–findings from the GerHIT multi-centre registry study. Thromb Res. 2014;134:29–35.
Tokgoz H, Caliskan U, Demir M. Successful use of fondaparinux in a child with heparin-induced thrombocytopenia. Blood Coagul Fibrinolysis. 2012;23:769–71.
Andrew M, Marzinotto V, Brooker LA, et al. Oral anticoagulation therapy in pediatric patients: a prospective study. Thromb Haemost. 1994;71:265–9.
Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 8th edn. Chest. 2008;133:160s–98s.
Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS. An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198:198–9.
Jansma B, Montgomery J, Dietrich S, Mixon MA, Peksa GD, Faine B. Emergent warfarin reversal with fixed-dose 4-factor prothrombin complex concentrate. Ann Pharmacother. 2020;54:1090–5.
Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–40.
Yarbrough PM, Varedi A, Walker A, Rondina MT. Argatroban dose reductions for suspected heparin-induced thrombocytopenia complicated by Child-Pugh class C liver disease. Ann Pharmacother. 2012;46:e30.
Oschman A. Survey results: characterization of direct thrombin inhibitor use in pediatric patients. J Pediatr Pharmacol Ther. 2014;19(1):10–5.
Moffett BS, Teruya J. Trends in parenteral direct thrombin inhibitor use in pediatric patients: analysis of a large administrative database. Arch Pathol Lab Med. 2014;138:1229–32.
Zaleski KL, DiNardo JA, Nasr VG. Bivalirudin for pediatric procedural anticoagulation: a narrative review. Anesth Analg. 2019;128:43–55.
Hursting MJ, Dubb J, Verme-Gibboney CN. Argatroban anticoagulation in pediatric patients: a literature analysis. J Pediatr Hematol Oncol. 2006;28:4–10.
Brandão LR, Albisetti M, Halton J, et al. Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood. 2020;135:491–504.
Payne RM, Burns KM, Glatz AC, et al. A multi-national trial of a direct oral anticoagulant in children with cardiac disease: design and rationale of the Safety of ApiXaban On Pediatric Heart disease On the preventioN of Embolism (SAXOPHONE) study. Am Heart J. 2019;217:52–63.
Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14.
Young G, Lensing AWA, Monagle P, et al. Rivaroxaban for treatment of pediatric venous thromboembolism. An Einstein-Jr phase 3 dose-exposure-response evaluation. J Thromb Haemost. 2020;18:1672–85.
Monagle P, Lensing AWA, Thelen K, et al. Bodyweight-adjusted rivaroxaban for children with venous thromboembolism (EINSTEIN-Jr): results from three multicentre, single-arm, phase 2 studies. Lancet Haematol. 2019;6:e500–9.
Levy G. Clinical pharmacokinetics of aspirin. Pediatrics. 1978;62:867–72.
Giglia TM, Massicotte MP, Tweddell JS, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease. Circulation. 2013;128:2622–703.
Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–91.
Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85.
Parodi G, Valenti R, Bellandi B, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601–6.
Tarango C, Manco-Johnson MJ. Pediatric thrombolysis: a practical approach. Front Pediatr. 2017;5:260–260.
Fox CK, Sidney S, Fullerton HJ. Community-based case–control study of childhood stroke risk associated with congenital heart disease. Stroke. 2015;46:336–40.
Domi T, Edgell DS, McCrindle BW, et al. Frequency, predictors, and neurologic outcomes of vaso-occlusive strokes associated with cardiac surgery in children. Pediatrics. 2008;122:1292–8.
Kumar K. Neurological complications of congenital heart disease. Indian J Pediatr. 2000;67:287–91.
Sun M-S, Jin H, Sun X, et al. Free radical damage in ischemia-reperfusion injury: an obstacle in acute ischemic stroke after revascularization therapy. Oxid Med Cell Longev. 2018;2018:3804979.
Jonas RA. Neurological protection during cardiopulmonary bypass/deep hypothermia. Pediatr Cardiol. 1998;19:321–30.
Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I-109–14.
Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–38.
Shah R, Nayyar M, Jovin IS, et al. Device closure versus medical therapy alone for patent foramen ovale in patients with cryptogenic stroke: a systematic review and meta-analysis. Ann Intern Med. 2018;168:335–42.
Baumgartner H, De Backer J. The ESC Clinical Practice Guidelines for the Management of Adult Congenital Heart Disease 2020. Eur Heart J. 2020;41:4153–4.
Meier B, Blaauw Y, Khattab AA, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. EuroIntervention. 2015;10:1109–25.
Mehta R, Lee KJ, Chaturvedi R, Benson L. Complications of pediatric cardiac catheterization: a review in the current era. Catheter Cardiovasc Interv. 2008;72:278–85.
Brotschi B, Hug MI, Kretschmar O, Rizzi M, Albisetti M. Incidence and predictors of cardiac catheterisation-related arterial thrombosis in children. Heart. 2015;101:948–53.
Bratincsák A, Moore JW, El-Said HG. Low dose tissue plasminogen activator treatment for vascular thrombosis following cardiac catheterization in children: a single center experience. Catheter Cardiovasc Interv. 2013;82:782–5.
Laurin S, Lundström NR. Venous thrombosis after cardiac catheterization in infants. Acta Radiol. 1987;28:241–6.
Ruud E, Natvig S, Holmstrøm H, Wesenberg F. Low prevalence of femoral venous thrombosis after cardiac catheterizations in children: a prospective study. Cardiol Young. 2002;12:513–8.
Hanslik A, Kitzmüller E, Thom K, et al. Incidence of thrombotic and bleeding complications during cardiac catheterization in children: comparison of high-dose vs. low-dose heparin protocols. J Thromb Haemost. 2011;9:2353–60.
Celermajer DS, Robinson J, Taylor J. Vascular access in previously catheterised children and adolescents: a prospective study of 131 consecutive cases. Heart. 1993;70:554–7.
Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2:3292–316.
Massicotte MP, Dix D, Monagle P, Adams M, Andrew M. Central venous catheter related thrombosis in children: analysis of the Canadian Registry of Venous Thromboembolic Complications. J Pediatr. 1998;133:770–6.
Streif W, Andrew ME. Venous thromboembolic events in pediatric patients: diagnosis and management. Hematol Oncol Clin North Am. 1998;12:1283–312.
Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877–905.
Yang JY, Williams S, Brandão LR, Chan AK. Neonatal and childhood right atrial thrombosis: recognition and a risk-stratified treatment approach. Blood Coag Fibrinol. 2010;21:301–7.
Bendaly EA, Batra AS, Ebenroth ES, Hurwitz RA. Outcome of cardiac thrombi in infants. Pediatr Cardiol. 2008;29:95–101.
Shaffer KM, Mullins CE, Grifka RG, et al. Intravascular stents in congenital heart disease: short- and long-term results from a large single-center experience. J Am Coll Cardiol. 1998;31:661–7.
Feltes TF, Bacha E, Beekman RH, 3rd, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2607–52.
Al Jubair KA, Al Fagih MR, Al Jarallah AS, et al. Results of 546 Blalock–Taussig shunts performed in 478 patients. Cardiol Young. 1998;8:486–90.
Li JS, Yow E, Berezny KY, et al. Clinical outcomes of palliative surgery including a systemic-to-pulmonary artery shunt in infants with cyanotic congenital heart disease: does aspirin make a difference? Circulation. 2007;116:293–7.
Fenton KN, Siewers RD, Rebovich B, Pigula FA. Interim mortality in infants with systemic-to–pulmonary artery shunts. Ann Thorac Surg. 2003;76:152–6.
Vongpatanasin W, Hillis LD, Lange RA. Prosthetic heart valves. N Engl J Med. 1996;335:407–16.
Cáceres-Lóriga FM, Pérez-López H, Santos-Gracia J, Morlans-Hernandez K. Prosthetic heart valve thrombosis: pathogenesis, diagnosis and management. Int J Cardiol. 2006;110:1–6.
Dangas GD, Weitz JI, Giustino G, Makkar R, Mehran R. Prosthetic heart valve thrombosis. J Am Coll Cardiol. 2016;68:2670–89.
Lengyel M, Fuster V, Keltai M, et al. Guidelines for management of left-sided prosthetic valve thrombosis: a role for thrombolytic therapy. J Am Coll Cardiol. 1997;30:1521–6.
Gürsoy MO, Kalçık, M, Yesin M, et al. A global perspective on mechanical prosthetic heart valve thrombosis: Diagnostic and therapeutic challenges. Anatol J Cardiol. 2016;16:980.
Gündüz S, Kalçık M, Gürsoy MO, Güner A, Özkan M. Diagnosis, treatment & management of prosthetic valve thrombosis: the key considerations. Expert Rev Med Dev. 2020;17:209–21.
Özkan M, Gündüz S, Gürsoy OM, et al. Ultraslow thrombolytic therapy: a novel strategy in the management of PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE: the Ultra-slow PROMETEE trial. Am Heart J. 2015;170:409–18.e1.
Khajali Z, Mohammadzadeh S, Maleki M, et al. Fibrinolytic therapy for mechanical pulmonary valve thrombosis. Pediatr Cardiol. 2015;36:171–6.
Ramos AI, Ramos RF, Togna DJ, et al. Fibrinolytic therapy for thrombosis in cardiac valvular prosthesis short and long term results. Arq Bras Cardiol. 2003;81:393–8.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–95.
De Caterina R, Husted S, Wallentin L, et al. Parenteral anticoagulants in heart disease: current status and perspectives (Section II). Position paper of the ESC Working Group on Thrombosis–Task Force on Anticoagulants in Heart Disease. Thromb Haemost. 2013;109:769–86.
Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH. Antithrombotic and thrombolytic therapy for valvular disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e576S–600S.
RJ IJ, Slieker MG, Hazekamp MG, et al. Mitral valve replacement with the 15-mm mechanical valve: a 20-year multicenter experience. Ann Thorac Surg. 2020;110:956–61.
Kiper C, Cua CL, Baker P, 3rd, McConnell P. Mitral valve replacement in pediatrics using an extracellular matrix cylinder valve: a case series. Pediatr Cardiol. 2020;41:1458–65.
Gillespie MJ, Benson LN, Bergersen L, et al. Patient selection process for the harmony transcatheter pulmonary valve early feasibility study. Am J Cardiol. 2017;120:1387–92.
Quinonez LG, Breitbart R, Tworetsky W, Lock JE, Marshall AC, Emani SM. Stented bovine jugular vein graft (Melody valve) for surgical mitral valve replacement in infants and children. J Thorac Cardiovasc Surg. 2014;148:1443–9.
Manlhiot C, Brandao LR, Kwok J, et al. Thrombotic complications and thromboprophylaxis across all three stages of single ventricle heart palliation. J Pediatr. 2012;161:513–19 e3.
Khairy P. Thrombosis in congenital heart disease. Expert Rev Cardiovasc Ther. 2013;11:1579–82.
Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:887S–968S.
Monagle P. Thrombosis in children with BT shunts, Glenns and Fontans. Prog Pediatr Cardiol. 2005;21:17–21.
Wells WJ, Yu RJ, Batra AS, Monforte H, Sintek C, Starnes VA. Obstruction in modified Blalock shunts: a quantitative analysis with clinical correlation. Ann Thorac Surg. 2005;79:2072–6.
Procelewska M, Kolcz J, Januszewska K, Mroczek T, Malec E. Coagulation abnormalities and liver function after hemi-Fontan and Fontan procedures—the importance of hemodynamics in the early postoperative period. Eur J Cardiothorac Surg. 2007;31:866–72.
Cheung EW, Chay GW, Ma ES, Cheung YF. Systemic oxygen saturation and coagulation factor abnormalities before and after the fontan procedure. Am J Cardiol. 2005;96:1571–5.
Odegard KC, McGowan FX Jr, Zurakowski D, et al. Coagulation factor abnormalities in patients with single-ventricle physiology immediately prior to the Fontan procedure. Ann Thorac Surg. 2002;73:1770–7.
van Nieuwenhuizen RC, Peters M, Lubbers LJ, Trip MD, Tijssen JG, Mulder BJ. Abnormalities in liver function and coagulation profile following the Fontan procedure. Heart. 1999;82:40–6.
Khairy P, Fernandes SM, Mayer JE, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85.
Varma C, Warr MR, Hendler AL, Paul NS, Webb GD, Therrien J. Prevalence of “silent” pulmonary emboli in adults after the Fontan operation. J Am Coll Cardiol. 2003;41:2252–8.
Deshaies C, Hamilton RM, Shohoudi A, et al. Thromboembolic risk after atriopulmonary, lateral tunnel, and extracardiac conduit Fontan surgery. J Am Coll Cardiol. 2019;74:1071–81.
Egbe AC, Connolly HM, McLeod CJ, et al. Thrombotic and embolic complications associated with atrial arrhythmia after Fontan operation: role of prophylactic therapy. J Am Coll Cardiol. 2016;68:1312–9.
Kawamatsu N, Ishizu T, Machino-Ohtsuka T, et al. Direct oral anticoagulant use and outcomes in adult patients with Fontan circulation: a multicenter retrospective cohort study. Int J Cardiol. 2021;327:74–9.
Al-Jazairi AS, Al Alshaykh HA, Di Salvo G, De Vol EB, Alhalees ZY. Assessment of late thromboembolic complications post-Fontan procedure in relation to different antithrombotic regimens: 30-years’ follow-up experience. Ann Pharmacother. 2019;53:786–93.
Iyengar AJ, Winlaw DS, Galati JC, et al. No difference between aspirin and warfarin after extracardiac Fontan in a propensity score analysis of 475 patients. Eur J Cardiothorac Surg. 2016;50:980–7.
Estes JW. The kinetics of heparin. Ann N Y Acad Sci. 1971;179:187–204.
Michelson AD, Bhatt DL. How I use laboratory monitoring of antiplatelet therapy. Blood. 2017;130:713–21.
Li JS, Yow E, Berezny KY, et al. Dosing of clopidogrel for platelet inhibition in infants and young children. Circulation. 2008;117:553–9.
Acknowledgements
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJ) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. The authors do not use funding or editorial assistance and the manuscript has not been presented or published previously. The tables and figures are original and have been produced by the authors (VR and EA) for the purpose of this manuscript.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contributions
Eman Abdelghani: manuscript concept design, literature review, and drafting of the manuscript. Jean Giver: manuscript review. Clifford Cua: manuscript review and editing. Vilmarie Rodriguez: concept design, literature review, writing and editing.
Funding
No funding or sponsorship was received for the study or publication of this article.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Abdelghani, E., Cua, C.L., Giver, J. et al. Thrombosis Prevention and Anticoagulation Management in the Pediatric Patient with Congenital Heart Disease. Cardiol Ther 10, 325–348 (2021). https://doi.org/10.1007/s40119-021-00228-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-021-00228-4