Abstract
Purpose
Reliable and comprehensive data on the HIV/AIDS and TB co-pandemics from Central Africa remain scarce. This systematic review provides a comprehensive overview on current and past research activities in the region and provides a basis for future research work to close knowledge gaps.
Methods
The scientific literature was searched for publications meeting the following search terms: “tuberculosis” or “HIV” or “acquired immunodeficiency syndrome”, combined with “Central Africa”, or the names of individual countries within the region. Original studies, reviews and case series were included, and a selection of relevant articles was made.
Results
Most research in the field of HIV and TB has been conducted in Cameroon, where the epidemics have been described fairly well. The Democratic Republic of Congo ranked second on the amount of publications, despite the civil wars over the past several decades. Very little has been published on HIV and TB in the other countries, possibly due to the poor infrastructure of health care systems, lack of scientific capacity building or shortage of laboratory equipment.
Conclusions
Despite the relatively high burden of HIV and TB in the Central African region, the amount of research activities on these topics is limited. A better understanding of the co-epidemics in this region is urgently needed. The occurrence of opportunistic infections, treatment complications and drug resistance in TB and HIV need to be better described; the failure of public health systems needs to be understood, and research infrastructure needs to be developed. Only then will it be possible to turn the tide against the HIV and TB epidemics in this region.
Similar content being viewed by others
Introduction
Thirty years after the emergence of HIV, huge investments have led to important achievements in its control; however, the virus still causes one of the biggest public health problems worldwide. The total number of people living with HIV is still increasing, mainly due to longer survival on antiretroviral therapy (ART) [1]. Globally, over 50 % of eligible patients now receive ART, and the number of new infections is declining [1]. Within the global scenario, sub-Saharan Africa carries the greatest HIV burden. In 2012, 61 % of all 35.3 million people living with HIV were living in this region [1].
Tuberculosis is the most frequent opportunistic infection and an important cause of morbidity and mortality in people living with HIV. TB–HIV co-infection rates vary throughout sub-Saharan Africa, but exceed 95 % in the extreme [2]. However, the percentage of notified TB patients tested for HIV varies widely; in Central Africa, according to the World Health Organization (WHO), the rates span from 17 % tested in the Republic of Congo (with 33 % being positive) to 82 % tested in Cameroon (with 37 % being positive) [3]. The worldwide incidence of TB was estimated to be 8.6 million cases in 2012, with 27 % of all tuberculosis patients living in sub-Saharan Africa [3]. In recent years, drug-resistant tuberculosis (DR-TB) has rapidly emerged as a problem of great concern, posing an important global health threat and adding to the challenges of under-resourced health care systems in heavily afflicted regions [4–6].
The Central African region, straddling the equator, is generally covered with dense primary rainforest and, therefore, often difficult to access. The following countries are defined as being part of this region: Cameroon, Chad, the Central African Republic (CAR), Republic of Congo (Congo Brazzaville), Democratic Republic of Congo (DRC), Equatorial Guinea and Gabon. Although HIV is thought to originate from this region [7–11], the area is left blank on many maps describing the epidemic, and if data are available, they are often inadequate [12–14]. The Central African region has one of the lowest doctor–patient ratios in the world [15], varying from 1:267 in Cameroon [16], 1:1,000 in Gabon (anecdotal information), up to 1:9,090 in DRC [17]. With the widespread lack of DOTS (directly observed treatment, short-course) implementation, cure rates are low and data collection and monitoring systems are underdeveloped. TB prevalence has been reported to be relatively stable, lower than the levels seen in Southern Africa, although this could partly be due to under-reporting [3]. Table 1 provides an overview of the available epidemiological data according to the WHO and UN [1, 3].
The purpose of this review is to systematically explore the scientific knowledge and current research activities on TB and HIV in the Central African region. Activities in several research fields within this region are evaluated and knowledge gaps identified, with a focus on epidemiologic research activities and clinical aspects of HIV and TB, HIV co-infections, functioning of treatment programmes, drug resistance and public health issues.
Methods
The PubMed database was searched using the MeSH terms for tuberculosis, HIV or acquired immunodeficiency syndrome. These terms were combined with the MeSH term for Central Africa [defined in the MeSH database as consisting of: Cameroon, Chad, the Central African Republic (CAR), Republic of Congo, Democratic Republic of Congo (DRC), Equatorial Guinea and Gabon]. The MeSH terms tuberculosis, HIV or acquired immunodeficiency syndrome were also directly combined with individual countries for ‘all fields’. Articles in English, French and Spanish were eligible if they were original studies, reviews or case series describing 10 cases or more. Case reports were excluded, as were articles published before 1991; articles published before this date were considered less relevant for the analysis of the current situation. Articles presenting data from clinical or epidemiological research focused on HIV and/or TB were selected and included based upon their title and abstract. Publications that were considered eligible were included for further analysis. In case there was no full text available, relevant abstracts were also included. The literature search was conducted up to February 2013. Relevant data were extracted and summarised in a separate file, categorised by country and subject, prior to the writing of this manuscript.
Results
Literature search
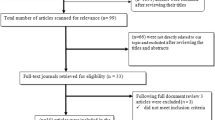
A search for ‘Central Africa’ and ‘HIV’ or ‘acquired immunodeficiency syndrome’ returned 820 articles. Using individual country names returned 245 articles for Cameroon, 11 for Equatorial Guinea, 8 for Chad, 69 for CAR, 290 for DRC, 59 for Congo Brazzaville and 45 for Gabon (total = 727). ‘Tuberculosis’ and ‘Central Africa’ returned 217 articles, and then with individual countries as follows: 60 for Cameroon, 5 for Equatorial Guinea, 12 for Chad, 23 for CAR, 54 for DRC, 26 for Congo–Brazzaville and 21 for Gabon (total = 201). After screening for relevance based on the title and abstract, a total of 130 articles describing HIV or TB in Central Africa remained for further review (Figs. 1 and 2).
Epidemiological research activities in Central African HIV and TB epidemics
With the HIV and TB epidemics being closely intertwined, Table 1 and Fig. 3 depict the epidemiological characteristics for both, as extracted from the 2013 WHO and UNAIDS reports [1, 3] (Table 1; Fig. 3).
Most of the regional HIV and TB research in the Central African region originates from Cameroon. Risk factors for HIV infection appear to be similar to those seen across sub-Saharan Africa, with HIV being more prevalent in women (56.3 %, sero-prevalence for women 6.3 % vs. 5.2 % for men) [18–20], in both rural and urban settings, whilst HIV-related mortality is higher in men (hazard ratio 1.73 [95 % CI 1.37–2.19]) [21]. Other known risk factors for HIV-related mortality were also observed, including a BMI <18.5, CD4+ cell count <50 cells/mm3 and anaemia (Hb <8.5 g/L) [21]. In rural settings, HIV prevalence varied substantially between villages, ranging from 0 to 18 % [20], with a higher prevalence among people who travel [22]. The prevalence of TB in Cameroon showed a steady increase together with the HIV epidemic, and increased HIV prevalence amongst pulmonary TB patients corresponded to the general rise in HIV in the total population [23]. TB is known to be more prevalent in prisons. A recent study showed a prevalence of active pulmonary TB (PTB) of 3.3 % in the central prison in Yaoundé [24], and 50 % of prisoners with active PTB were not diagnosed by the prison TB control programme [24].
For Equatorial Guinea, there were no publications found on HIV or TB over the past 5 years. Only one publication on HIV was found, reporting a sero-prevalence among healthy people living in a rural area of 12/2042 (0.6 %) in 1999 [25]. One observational study in molecular epidemiology showed clustering of TB strains in 61.6 %, suggesting a high degree of recent transmission [26].
In Chad, the sero-prevalence of HIV in a population-wide survey was 11.1 % [27]. A health economy study estimated the cumulative costs attributable to AIDS at $836 for the household per patient, whereas the gross national product was estimated at $200 per capita [28]. In Chad, several studies on TB have been carried out in nomadic pastoralists (6 % of the total population) [29, 30]; in one of these studies, TB was suspected in 4.6 % [29].
CAR has the highest adult HIV prevalence in francophone sub-Saharan Africa, although rates vary highly by region (from 2.7 to 30.7 %) [31]. In the CAR, TB incidence was estimated to be 250/100,000 [32]. Mortality rates for HIV-positive TB patients were at least tripled compared to HIV-negative TB patients [33].
In the DRC, the civil wars have inevitably impacted heavily on the health care system. A study in 2001 among 7,277 blood donors (in the pre-screening era) found a HIV-1 prevalence of 6.4 % [34]. The prevalence of HIV amongst women living in internally displaced person camps was up to twice as high as the prevalence amongst women from river populations (3.1 % vs. 7.6 %) [35]. Risk factors for infection with HIV in the DRC were found to be living in an urban area; polygamy (which is common); having a sexually transmitted infection (STI); and being paid for sex [35–39]. In an autopsy study, TB was defined as the most frequent cause of death in AIDS patients [40]. A more recent retrospective study analysing hospital registers confirmed that HIV–TB was linked with high mortality [41]. Paediatric HIV was found to be an important risk factor for death from diarrhoea [42] and neurodevelopment was significantly slower in HIV-infected children [43]. Also in the DRC, the incidence of TB is higher in HIV-positive patients; 2.4/100 person years versus 0.38/100 person years in sero-negative individuals in a survey in 2000 [44]. Initiating ART is an efficient intervention to decrease TB-related mortality (estimated reduction of mortality of 36 %) [45] and to suppress the incidence of TB, even in HIV-infected children. ART reduced the hazard of developing TB in children by 50 % [46]. However, household contact surveys in Kinshasa found similar rates of transmission to household contacts of HIV-positive and -negative patients [47]. The estimated case notification rate in the DRC is 39 %. The TB–HIV co-infection rate has been reported to be 11 % and the prevalence of multi-drug resistance in new cases as 1.7 % [17]. The difficulties met in improving health care are demonstrated by the shortages of capacity: per 1,000 inhabitants of the DRC, there are 0.11 physicians and 0.53 nurses [17].
From the Republic of Congo, not much was published on the features of the HIV epidemic over the past several years. However, AIDS was the most important cause of death in two morgue-based studies [48, 49]. Mortality was significantly higher in women, and in the age group of 25–44 years, mortality was tripled by AIDS in 2000. A few studies report on TB in the Republic of Congo, mainly on paediatric TB [50–53]. The civil war from 1997–1999 aggravated the TB epidemic; after the war, the number of TB cases had doubled and case notification dropped from 100 to 76 % [54]. TB rates in hospitalised children increased to 20 % in 2000, also illustrating the serious consequences of the civil wars [52, 53]. HIV co-infection was reported in 43–65 % of all paediatric TB patients, and mortality due to TB was mainly found in HIV co-infected children [50, 51, 55].
Gabon is home to many immigrants from its neighbouring and West African francophone countries. Serotypes of HIV are very variable and even HIV-2 is imported (4 % of all HIV infections in a study in 1998) [56]. Over the past decades, there was an increase in the prevalence of HIV; HIV prevalence in pregnant women was 1.26 % in 1993 [57]; by 2008, this prevalence had increased to 6.0 % [58]. In Gabon, STIs are more common among immigrants [59]. HIV prevalence was found to be higher in primarily infertile women [60]. In Gabon, DOTS was not successfully implemented, due to problems with drug supply [61]. HIV co-infection rates vary between 26 % [62] and 46 % [63]. About 20 % of all hospital admissions in the principal hospital in the capital Libreville are attributable to TB [64].
Clinical and diagnostic aspects
HIV
Not much has been published on the clinical status on presentation of HIV patients in the Central African region.
In Cameroon, the predominant presenting clinical symptoms of HIV were persistent fever, chronic diarrhoea and excessive weight loss [19]. A recent study in the Western region showed a median baseline CD4 count of 104 cells/mm3, and most patients presented at clinical stage A3 (54.4 %) or B3 (40.4 %) [65]. As in other parts of the world, dermatological problems are very common; 68.8 % of a cohort of HIV patients in Cameroon reported at least one skin problem, with generalised pruritus and oral candidiasis being the most common [18]. Also, ocular complications of HIV are frequent; in a cohort of 57 patients, 63.2 % had a pathological eye examination, with herpetic keratitis and ophthalmic herpes zoster being the most common [66].
To the best of our knowledge, at the time of writing, there are currently PubMed listed publications available on the clinical presentation or therapeutic response of HIV patients in Equatorial Guinea, Chad, CAR or the DRC. The most frequent symptoms in elderly patients (>55 years) in Congo between 1990 and 1996 were weight loss, fever, diarrhoea, neuro-psychiatric disorders and respiratory symptoms [67]. In this period, mortality was still tremendously high; 74 % of 175 patients deceased after 1 year and after 2 years, all patients had died. In children, the main symptoms were similar; weight loss, persistent fever and chronic diarrhoea [55]. The most frequent opportunistic infection described was TB [55]. In Gabon, in 2007, in a cross-sectional study in 749 patients, most patients presented with a relatively advanced disease, classified as WHO stage B (33.5 %) and stage C (27.1 %) [68]. Opportunistic infections were observed in 95 % of cases; 57 % fungal, 30.7 % bacterial and 7.3 % viral [68]. The most frequent opportunistic infections were oral candidiasis, herpes zoster, salmonellosis and TB [68].
TB
With regard to the diagnostics and therapy of TB, there are still many problems to be addressed in the Central African region. Culture and drug sensibility testing are not available in many areas, let alone the expensive second-line drugs to treat drug-resistant TB.
The reported sero-prevalence of HIV in TB patients in Cameroon increased from around 10–15 % in the 1990s [69, 70] to 35–40 % a decade later [71, 72]. Constitutional symptoms were seen more often in HIV-infected patients [71] and pregnancy. HIV infection and diabetes mellitus were risk factors of atypical presentations such as lower lung field TB [69]. Two publications were found describing treatment response; a cure rate of 69 % was reported and a default rate of 23.6 %, which was higher in HIV co-infected patients [72]. The mortality rate (3.3 %) was higher in HIV co-infected patients [72]. A considerable proportion (13.4 %) of new pulmonary TB patients had a non-conversion of their sputa after 2 months of treatment [73].
No publications were found relating to diagnostic or therapeutic aspects of TB for Equatorial Guinea, Chad or the Republic of Congo. In CAR, in the 1990s, the diagnosis of TB was mostly based on clinical and radiologic findings; a retrospective study reported that sputum smears were available for only 52 % of patients (42 % was positive) [74]. This same study reported a high prevalence of HIV in TB patients; 82 % of those tested. Patients presented late to the hospital; 11 % deceased in the first week of hospitalisation [74]. A recent prospective study among 66 HIV-positive pulmonary TB patients reported that only 44 % had a positive sputum smear [75]; sputum smear negativity was associated with other respiratory tract infections, but was less frequent with CD4 counts <50 cells/μL [75].
A recent retrospective analysis in the DRC reported a mortality rate of 6.8 %; 53 % of these patients deceased during the first 2 months of treatment [45]. Mortality was high in HIV co-infected patients and patients with extra-pulmonary TB (EPTB), and the risk decreased with a timely start of ART [45].
For Gabon, all publications found were retrospective studies with rather small numbers of patients (16–156 patients). Even in Gabon, one of the more wealthy countries of Africa, there is no facility yet performing routine sputum culture and sensitivity testing. HIV patients present more often with constitutional symptoms and EPTB [76]. Around 48 % of patients present with EPTB [64]. Diagnostic difficulties are illustrated by a study on lymph node TB in the capital where only 57.8 % of suspected patients underwent lymph node biopsy to confirm the diagnosis [63]. The number of patients lost to follow up is high, around 40 %, mostly due to financial constraints, since patients had to pay for their medication themselves [61, 63, 64, 77].
Co-infections with HIV, MTB and other pathogens
Aside HIV and TB, many other infectious diseases also cause important morbidity in the Central African region, and co-infections with HIV or TB complicate the problem.
A recent study on HIV–malaria co-infection estimated the HIV prevalence in Cameroon to be 5.4 % [78]. Infection with intestinal parasites was investigated in Equatorial Guinea; 212/260 (81.5 %) of HIV-positive individuals were infected with one or more intestinal helminths or pathogenic protozoa, compared to only 37/50 (74 %) of HIV-negative controls [79].
In CAR as well as in Chad, a relationship was found between HIV and HSV-2 infection; there was a co-infection rate of 22 %, which was significantly higher compared to other STDs [27, 80, 81]. HIV infection is an important risk factor for meningitis in the CAR (77 % of all patients with meningitis in a hospital-based study were HIV-infected), and meningitis in HIV patients is caused by cryptococcal infection in 39 % of cases [82]. Mortality from all types of meningitis is high in HIV patients (57 %) [82]. HIV-1-infected mothers were more frequently exposed to placental malaria [83].
In Gabon, HIV as well as HBV, HCV and HTLV-1 are endemic; all are risk factors to develop B-lympho-proliferative disorders [84]. A study in 1991 found a prevalence of HTLV-1 of 6.8 % in an adult female population [60].
HIV and TB control programmes in the region
ART roll-out and prevention of mother-to-child transmission
With regard to ART roll-out and prevention of mother-to-child transmission, the Central African region has a relatively low coverage compared to other areas [1].
Whereas in 2002 only 2.8 % of eligible patients in Yaoundé received ART [19], this increased to 33 % (of male patients) and 67 % (of female patients) in 2010 [85]. However, in a recent study in 398 (HIV-positive and -negative) pregnant women, only 27 % of the HIV-positive patients were on ART [86]. The prevention-of-mother-to-child-transmission programme requires additional support; a cross-sectional analysis investigating dried blood spots of 14,763 infants born to HIV-positive mothers in remote areas showed an infant infection rate of 1,452/14,763 (12.3 %), mounting up to 17 % in certain areas in 2010 [87]. This percentage is remarkably higher than reported transmission rates in other countries in sub-Saharan Africa, where it is usually below 10 % [88–94]. This is particularly worrisome, as Cameroon has the second best ART coverage in pregnant women in the region, with 64 %, compared to 19 % in Congo, 14 % in Chad and 13 % in DRC; only Gabon seems to perform better, with 70 % coverage reported [1].
For therapeutic aspects, except for HIV drug resistance (see later paragraph), other resistances need to be taken into account. A cross-sectional survey in co-infected patients in Yaoundé in 2012 showed hepatitis B virus resistance to lamivudine of 14 and 60 %, respectively, after 12 and 24 months of ART (without tenofovir) [95]. Regarding the monitoring of treatment, a recent non-inferiority trial (one of the few randomised trials from the region) showed the inferiority of clinical monitoring alone versus clinical monitoring combined with immunological follow up with CD4 count and viral loads [96]. However, the authors conclude that clinical monitoring alone can be used in the initial phase of scale-up of ART programmes, if the necessary laboratory infrastructure is not available.
A recent study in rural DRC identified the lack of HIV testing as the main bottleneck for the low coverage of ART in the country, whereas the dysfunction of the referral system between prevention of mother-to-child transmission and TB programmes to ART programmes is problematic [97]. Condom promotion was found to be an effective intervention to prevent transmission [38, 98]. The DRC is amongst the countries with the lowest ratio of eligible patients who receive ART; only 32 % of people living with HIV are entering HIV care (41 % in urban areas vs. 11 % in rural areas) [97].
Adherence, therapeutic response to ART and integration of TB/HIV care
Adherence and therapeutic response to ART are crucial in curbing the epidemic. Nevertheless, not much has been published on these issues in the Central African region.
In Cameroon, patient as well as health care-associated interruption of ART has been reported to be a frequently encountered problem [99, 100]. It is related to pharmacy shortages, patients having to pay for the drugs themselves, binge drinking and ‘slimming’ symptoms causing incompliance [100]. A recent retrospective analysis of 40 ART clinics showed high rates of patients lost to follow up; only 20 % of clinics achieved the target to decrease the proportion of patients lost to follow up to less than 20 % [101]. Adherence was found to be better in patients who presented with higher CD4 cell counts at baseline, and after longer treatment intervals [65]. Patient-related reasons for non-adherence were loss of memory, work, sleep, non-compliance during travel and lack of food [65]. Another study found 67/112 (60 %) and 79/119 (66 %) of patients being adherent to their ART at months 6 and 12, respectively [102]. Before ART was available for free, financial constraints impaired access to ART; 69 % of 84 patients were willing to pay $1 a day, but this decreased to 22 % for $2 a day and only 9 % for $3 a day; in this study, 72 % of patients abandoned their treatment within 6 months [103]. Inconsistent condom use was reported by a considerable proportion of patients (50 % at month 6 and 59 % at month 12), whereas it decreased in patients with good adherence when compared to baseline [102]. Counselling needs to be improved, as a study in western Cameroon demonstrated that knowledge of HIV transmission and prevention was fair in only 64 % of HIV patients [65]. Twenty-nine percent of patients went to traditional healers before presenting to the clinic, thus illustrating the important role of traditional medicine in the region [65]. With regard to the implementation of TB–HIV collaborative activities, much can be improved in Cameroon. Only 61 % of TB patients were tested for HIV [104], and stigma-related reasons for declining HIV testing were frequent [105]. Also, according to counsellors’ perspectives, there is still an influence of certain traditional healers who convince the public that they can provide a permanent cure for HIV [105]. The implementation of the Global Fund Grant Round for TB (focusing on capacity building for TB diagnosis and management and laboratory strengthening) was investigated in the North-West region of Cameroon. Initially, this had a large impact by increasing case notification and detection by 50 %, whereas treatment success improved by 20 % [104]. However, at the end of the study, 2 years after the grant period had expired, the outcome indicators decreased again, possibly due to staff turnover. During this study interval (2003–2008), HIV testing for all TB patients was implemented; a TB–HIV co-infection rate of 53 % was found; in 1995, this was reported to be 9.9 % [106]. Qualitative research interviews showed the importance of trust in health care workers and family support for TB patients to get tested [105].
No publications were found on public health-related issues in Equatorial Guinea, Chad or the Republic of Congo. A prospective study in TB patients in CAR in the period 2002–2005 reported that there was no ART available, and only 12 % of 66 TB patients were aware of their HIV status [75].
A single publication evaluating the ART programme in Gabon was found; this study in Libreville reported 749 patients to be actively followed in 2007, of which 436 were on ART [68]. There was a decrease of mortality from 39 % during the first 6 months of follow up to 2.7 % after 2.5 years. However, there were no data on the number of patients lost to follow up.
HIV subtypes and HIV drug resistance
HIV subtypes
The variety of HIV strains is high in the Central African region and has been thoroughly described. This wide variety has been attributed to the origin of the HIV epidemics lying in this region. Most literature has been published on HIV variance in Cameroon. In Cameroon, the most frequent subgroup of HIV is group M, with group N and HIV-2 being very rare [107–109]. CRF02_AG is reported to be the most frequent recombinant form [110–119], although in the Eastern Region, CRF11_cpx was found to be more common, resembling the epidemics in the bordering countries, Chad and Central Africa [114]. In Equatorial Guinea, subtype A was the most frequent, but CRF02_AG was also common [120]. In Chad, CRF11_cpx is the most common [121, 122]. In the DRC, subtype A predominates [121, 123–125]. Mosaic strains were more common in female sex workers, whilst military personnel was more at risk to be infected with diverse strains [123]. Also in the Republic of Congo, subtype A was the most frequent [126–128]. CRF02_AG was found to be the most prevalent in Gabon [57, 129, 130].
HIV drug resistance
The only country in the Central African region where accurate data on HIV drug resistance are published is Cameroon. Recent data raise concerns with respect to the development of resistance to any of the first-line drugs, as seen in >35 % of patients on ART [131–133]. Resistance mutations are also seen in treatment-naïve individuals, suggesting that mutated viruses are transmitted as well. Resistance-conferring polymorphisms were found in 12 % of ART-naïve adult patients in a small recent study [16], and between 4.9 and 8.2 % in ART-naïve children [134, 135]. In children with treatment failure, resistance to ART was found in 90 % of patients [135]. Resistance to protease inhibitors (PIs) was 5.7 % in ART-naïve individuals versus 38.6 % in patients exposed to PIs [134].
In a cohort in Chad, 64 % of 88 followed patients on ART had at least one resistance mutation and 64 % showed virologic failure [121]. Recent reports from the CAR also indicate an alarming situation; ART failure has been reported in 30 % of adults, whilst there is no second-line treatment available in the country [136]. Drug resistance mutations were found in 13.9 % of ART-naïve children [137]. Another study reported virologic failure in 40 % of paediatric patients after 18 and 30 months of ART [138]. The most common resistance profiles were associated with the wide use of 3TC and first-generation non-nucleoside reverse transcriptase inhibitors (NNRTIs), as recommended by the WHO as the first-line treatment in sub-Saharan Africa.
One small study in Gabon found, in 11/19 (58 %) of samples, major mutations inevitably leading to drug resistance in patients after a mean of 17.7 months of ART exposure [139]. However, in untreated populations, grades of resistance were low (2.8 % in pregnant women) [57, 130] according to the authors because of low accessibility to ART [130].
No publications were found on HIV drug resistance in Equatorial Guinea, DRC and the Republic of Congo.
TB diagnosis and drug resistance
TB drug resistance
Data on TB drug resistance from the Central African region are scarce, as it is the case in most areas where TB culture is still not widely possible. Consequently, most articles are based on case series or small cohorts.
Reported rates for multi-drug-resistant (MDR) TB are moderate in the Central African region. A study in Cameroon reported the TB prevalence in sputum smear-positive patients as being 6.67 % in the Western region in 2009, whereas resistance to one drug was 13.3 % [140]. Throughout the whole region, mono-resistances to isoniazid and streptomycin, respectively, are the most frequent [26, 106, 140–142]. In Chad, no streptomycin resistance was found in a prospective study at the national referral hospital in 357 patients [143]. A more recent study in 232 cases in Chad reported 20 % mono-resistance in treatment-naïve TB patients, with resistance to isoniazid and streptomycin being most common, and 2.2 % MDR-TB [144]. In the CAR, resistance rates in children have been reported to be similar to adult rates; the overall drug resistance was 15.2 % and multi-drug resistance was 0.6 % in a study in Bangui in 2000 [142]. Another study showed that more than half of the studied MDR-TB strains (32/53) were new infections [31]. However, there was a low level of primary resistance in Bangui and Bimbo (CAR) in 2011, 14.7 %, with 0.4 % MDR-TB [145]. A study on MTB resistance in Kinshasa between 307 newly diagnosed TB patients in 2007 showed a primary resistance rate of 43.5 % to first-line drugs and 5.3 % MDR-TB [146]. For Gabon, only one case series was available reporting on resistant TB; 16 MDR and 3 extensively drug-resistant (XDR) cases were found between 2006 and 2010, of which only three were cured, as the patients had to pay for the medication themselves and there are few second-line drugs available in the country [147].
With the interpretation of all prevalence rates regarding TB drug resistance in the Central African region, the fact that infrastructure to perform adequate drug sensitivity testing is most often not available ought to be considered. Thus, further studies are needed in order to get an impression of the real situation in the Central African region with regard to TB drug resistance.
Discussion
Knowledge gaps
Although seemingly originating from this area, the HIV epidemic has been poorly addressed in terms of research in the Central African region. TB is highly endemic, but the features of the epidemic have been inadequately investigated.
Most research in the field of HIV and TB has been conducted in Cameroon, where the epidemics have been described fairly well. DRC ranked second on the amount of publications describing the TB and HIV epidemic, despite the civil wars over the past several decades, possibly due to the fact that the country has a large population. Very little has been published on HIV and TB in the other countries in the Central African region. This might be, in part, due to the poor infrastructure of health care systems, lack of scientific capacity building or shortage of equipment such as, for example, material for HIV or TB drug sensitivity testing.
In contrast, the wide variability of HIV subtypes in the Central African region has been well described. This is of scientific interest especially because of the hypothesis that the origin of the HIV pandemic lies somewhere in the Central African rainforest, and with the eye on vaccine development. However, a direct clinical implication of the subtypes still remains to be established.
A better description of drug resistance of HIV, but even more importantly of TB, is urgently needed within the Central African region, and a common view on both infections needs to be developed from all aspects, ranging from epidemiology to case treatment and control on the population level. In order to be able to improve patient care, it is of utmost importance to gain a solid overview of the current state of the HIV and TB epidemics in general within this region. Only then can obstacles within the arena of public health care be identified and overcome, and interventions be successfully and sustainably implemented.
Conclusions
This review illustrates the considerable burden of HIV and TB infections in the Central African region, and whilst basic epidemiological data as reported by the WHO and UNAIDS [1, 3] provide an insight into the dimension of the problem (which is substantial, although luckily not reaching the dimensions of the problem in the southernmost part of Africa), there continues to be a lack of systematic research (beyond Cameroon) within this area. Apart from the public health care systems which need to be improved in the region, in the field of infectious diseases, it is eminently important to see epidemics in a global perspective. Pathogens can act differently in distinct settings, influenced by, for example, geographical, environmental, genetic and societal variations. Therefore, a better understanding of the features of the HIV and TB epidemics in the Central African region are urgently needed. Implementation of improved diagnostics deserves attention.
The occurrence of opportunistic and other accompanying infections, treatment complications such as the immune reconstitution syndrome and the prevalence of drug resistance in TB and HIV need to be better described. Interventions in the public health care systems need to be evaluated after implementation, and research infrastructure needs to be developed. Only then will it be possible to turn the tide fully against the HIV and TB epidemics in the Central African region.
References
UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. 2013. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
John MA, Menezes CN, Chita G, Sanne I, Grobusch MP. High tuberculosis and HIV coinfection rate, Johannesburg. Emerg Infect Dis. 2007;13:795–6.
World Health Organization (WHO). Global tuberculosis report 2013. 2013. http://www.who.int/tb/publications/global_report/en/.
Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–807.
Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80.
Grobusch MP. Drug-resistant and extensively drug-resistant tuberculosis in southern Africa. Curr Opin Pulm Med. 2010;16:180–5.
Fonjungo PN, Mpoudi EN, Torimiro JN, Alemnji GA, Eno LT, Nkengasong JN, et al. Presence of diverse human immunodeficiency virus type 1 viral variants in Cameroon. AIDS Res Hum Retroviruses. 2000;16:1319–24.
Parris GE. Mechanism and history of evolution of symbiotic HIV strains into lethal pandemic strains: the key event may have been a 1927 trial of pamaquine in Leopoldville (Kinshasa), Congo. Med Hypotheses. 2007;69:838–48.
Strickland GT. An epidemic of hepatitis C virus infection while treating endemic infectious diseases in Equatorial Africa more than a half century ago: did it also jump-start the AIDS pandemic? Clin Infect Dis. 2010;51:785–7.
Vangroenweghe D. The earliest cases of human immunodeficiency virus type 1 group M in Congo-Kinshasa, Rwanda and Burundi and the origin of acquired immune deficiency syndrome. Philos Trans R Soc Lond B Biol Sci. 2001;356:923–5.
Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, et al. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008;455:661–4.
UNAIDS. UNAIDS report on the global AIDS epidemic 2010. 2010. http://www.unaids.org/globalreport/global_report.htm.
World Health Organization (WHO). Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. 2010. http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf.
Abubakar I, Zignol M, Falzon D, Raviglione M, Ditiu L, Masham S, et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13:529–39.
Sibanda EN, Stanczuk G, Kasolo F. HIV/AIDS in Central Africa: pathogenesis, immunological and medical issues. Int Arch Allergy Immunol. 2003;132:183–95.
Aghokeng AF, Kouanfack C, Laurent C, Ebong E, Atem-Tambe A, Butel C, et al. Scale-up of antiretroviral treatment in sub-Saharan Africa is accompanied by increasing HIV-1 drug resistance mutations in drug-naive patients. AIDS. 2011;25:2183–8.
Mauch V, Weil D, Munim A, Boillot F, Coninx R, Huseynova S, et al. Structure and management of tuberculosis control programs in fragile states—Afghanistan, DR Congo, Haiti, Somalia. Health Policy. 2010;96:118–27.
Mbuagbaw J, Eyong I, Alemnji G, Mpoudi N, Same-Ekobo A. Patterns of skin manifestations and their relationships with CD4 counts among HIV/AIDS patients in Cameroon. Int J Dermatol. 2006;45:280–4.
Mbanya DN, Zebaze R, Minkoulou EM, Binam F, Koulla S, Obounou A. Clinical and epidemiologic trends in HIV/AIDS patients in a hospital setting of Yaounde, Cameroon: a 6-year perspective. Int J Infect Dis. 2002;6:134–8.
Nyambi P, Zekeng L, Kenfack H, Tongo M, Nanfack A, Nkombe I, et al. HIV infection in rural villages of Cameroon. J Acquir Immune Defic Syndr. 2002;31:506–13.
Sieleunou I, Souleymanou M, Schönenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009;14:36–43.
Lydié N, Robinson NJ, Ferry B, Akam E, De Loenzien M, Abega S; Study Group on Heterogeneity of HIV Epidemics in African Cities. Mobility, sexual behavior, and HIV infection in an urban population in Cameroon. J Acquir Immune Defic Syndr. 2004;35:67–74.
Noeske J, Kuaban C, Cunin P. Are smear-positive pulmonary tuberculosis patients a ‘sentinel’ population for the HIV epidemic in Cameroon? Int J Tuberc Lung Dis. 2004;8:346–51.
Noeske J, Ndi N, Mbondi S. Controlling tuberculosis in prisons against confinement conditions: a lost case? Experience from Cameroon. Int J Tuberc Lung Dis. 2011;15:223–7.
Basaras M, Santamaría A, Sarsa M, Gutiérrez E, de Olano Y, Cisterna R. Seroprevalence of hepatitis B and C, and human immunodeficiency type 1 viruses in a rural population from the Republic of Equatorial Guinea. Trans R Soc Trop Med Hyg. 1999;93:250–2.
Tudó G, González-Martín J, Obama R, Rodríguez JM, Franco JR, Espasa M, et al. Molecular epidemiology of tuberculosis in the Bata and Malabo districts of Equatorial Guinea. Int J Tuberc Lung Dis. 2004;8:1458–63.
Charpentier C, Koyalta D, Ndinaromtan M, Tchobkréo B, Jenabian MA, Day N, et al. Distribution of HIV-1 and HSV-2 epidemics in Chad revealing HSV-2 hot-spot in regions of high-risk HIV spread. J Infect Dev Ctries. 2011;5:64–7.
Wyss K, Hutton G, N’Diekhor Y. Costs attributable to AIDS at household level in Chad. AIDS Care. 2004;16:808–16.
Daugla DM, Daoud S, Tanner M, Zinsstag J, Schelling E. Morbidity patterns in three nomadic communities in Chari-Baguirmi and Kanem, Chad. Med Trop (Mars). 2004;64:469–73.
Schelling E, Daoud S, Daugla DM, Diallo P, Tanner M, Zinsstag J. Morbidity and nutrition patterns of three nomadic pastoralist communities of Chad. Acta Trop. 2005;95:16–25.
Médecins Sans Frontières. Central African Republic: a state of silent crisis. 2011. http://www.msf.org.uk/sites/uk/files/a_state_of_silent_crisis_en_0.pdf.
Nouvel LX, Kassa-Kelembho E, Dos Vultos T, Zandanga G, Rauzier J, Lafoz C, et al. Multidrug-resistant Mycobacterium tuberculosis, Bangui, Central African Republic. Emerg Infect Dis. 2006;12:1454–6.
Garin B, Glaziou P, Kassa-Kelembho E, Yassibanda S, Mbelesso P, Morvan J. High mortality rates among patients with tuberculosis in Bangui, Central African Republic. Lancet. 1997;350:1298.
Mbendi Nlombi C, Longo-Mbenza B, Mbendi Nsukini S, Muyembe Tamfum JJ, Situakibanza Nanituma H, Vangu Ngoma D. Prevalence of HIV and HBs antigen in blood donors. Residual risk of contamination in blood recipients in East Kinshasa, Democratic Republic of the Congo. Med Trop (Mars). 2001;61:139–42.
Kim AA, Malele F, Kaiser R, Mama N, Kinkela T, Mantshumba JC, et al. HIV infection among internally displaced women and women residing in river populations along the Congo River, Democratic Republic of Congo. AIDS Behav. 2009;13:914–20.
Bernal MC, Galán MI, Ocete MD, Leyva A, García F, García-Valdecasas J, et al. A seroepidemiological study of human immunodeficiency virus infection in northeast Zaire. Infection. 1994;22:174–7.
Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102.
Laga M, Alary M, Nzila N, Manoka AT, Tuliza M, Behets F, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344:246–8.
Mwandagalirwa K, Jackson EF, McClamroch K, Bollinger R, Ryder RW, Weir SS. Local differences in human immunodeficiency virus prevalence: a comparison of social venue patrons, antenatal patients, and sexually transmitted infection patients in eastern kinshasa. Sex Transm Dis. 2009;36:406–12.
Nelson AM, Perriëns JH, Kapita B, Okonda L, Lusamuno N, Kalengayi MR, et al. A clinical and pathological comparison of the WHO and CDC case definitions for AIDS in Kinshasa, Zaïre: is passive surveillance valid? AIDS. 1993;7:1241–5.
Tshikuka Mulumba JG, Atua Matindii B, Kilauzi AL, Mengema B, Mafuta J, Eloko Eya Matangelo G, et al. Severity of outcomes associated to types of HIV coinfection with TB and malaria in a setting where the three pandemics overlap. J Community Health. 2012;37:1234–8.
Thea DM, St Louis ME, Atido U, Kanjinga K, Kembo B, Matondo M, et al. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. N Engl J Med. 1993;329:1696–702.
Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–8.
Ryder RW, Batter V, Kaseka N, Behets F, Sequeira D, M’Boly E, et al. Effect of HIV-1 infection on tuberculosis and fertility in a large workforce in Kinshasa, Democratic Republic of the Congo. AIDS Patient Care STDS. 2000;14:297–304.
Henegar C, Behets F, Vanden Driessche K, Tabala M, Bahati E, Bola V, et al. Mortality among tuberculosis patients in the Democratic Republic of Congo. Int J Tuberc Lung Dis. 2012;16:1199–204.
Edmonds A, Lusiama J, Napravnik S, Kitetele F, Van Rie A, Behets F. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol. 2009;38:1612–21.
Klausner JD, Ryder RW, Baende E, Lelo U, Williame JC, Ngamboli K, et al. Mycobacterium tuberculosis in household contacts of human immunodeficiency virus type 1-seropositive patients with active pulmonary tuberculosis in Kinshasa, Zaire. J Infect Dis. 1993;168:106–11.
Le Coeur S, Halembokaka G, Khlat M, Brouard N, Purhuence F, M’Pelé P, et al. Impact of AIDS on adult mortality: a morgue-based study in Pointe-Noire, Republic of Congo. AIDS. 2005;19:1683–7.
Pictet G, Le Coeur S, M’Pelé P, Brouard N, Lallemant M. Contribution of AIDS to the general mortality in Central Africa: evidence from a morgue-based study in Brazzaville, Congo. AIDS. 1998;12:2217–23.
Mabiala-Babela JR, M’Pemba Loufoua AB, Mouko A, Senga P. Pulmonary tuberculosis in infants in Brazzaville, Congo. A review of 117 cases. Med Trop (Mars). 2008;68:167–72.
Mabiala-Babela JR, Makosso E, Senga P. Retrospective study of 61 cases of multifocal tuberculosis in children in Brazzaville, Congo. Med Trop (Mars). 2008;68:41–4.
Mpemba Loufoua-Lemay AB, Nzingoula S. Peritoneal tuberculosis in children at the University Hospital Center of Brazzaville: 35 cases. Arch Pediatr. 2003;10:356–7.
M’Pemba Loufoua-Lemay AB, Youndouka JM, Pambou B, Nzingoula S. Child tuberculosis at the teaching hospital of Brazzaville from 1995 to 2003. Bull Soc Pathol Exot. 2008;101:303–7.
M’Boussa J, Yokolo D, Pereira B, Ebata-Mongo S. A flare-up of tuberculosis due to war in Congo Brazzaville. Int J Tuberc Lung Dis. 2002;6:475–8.
M’Pemba Loufoua Lemay AB, Mabiala Babela JR, Bantsimba T, Nzingoula S. Tuberculosis and HIV/AIDS co-infection in children: experience carried out in paediatric service of the teaching Hospital of Brazzaville, Republic of Congo (1995–2004). Bull Soc Pathol Exot. 2007;100:51–2.
Tevi-Benissan C, Okome M, Makuwa M, Nkoume MN, Lansoud-Soukate J, Georges A, et al. HIV-2 infection and HIV-1/HIV-2 dual reactivity in patients with and without AIDS-related symptoms in Gabon. Emerg Infect Dis. 1998;4:130–1.
Mounanga M, Revangue BS. HIV-1 seroprevalence in pregnant women in Libreville (Gabon). J Gynecol Obstet Biol Reprod (Paris). 1993;22:509–12.
Caron M, Lekana-Douki SE, Makuwa M, Obiang-Ndong GP, Biba O, Nkoghé D, et al. Prevalence, genetic diversity and antiretroviral drugs resistance-associated mutations among untreated HIV-1-infected pregnant women in Gabon, Central Africa. BMC Infect Dis. 2012;12:64.
Bertherat E, Georges-Courbot MC, Nabias R, Georges AJ, Renaut A. Seroprevalence of four sexually transmitted diseases in a semi-urban population of Gabon. Int J STD AIDS. 1998;9:31–6.
Schrijvers D, Delaporte E, Peeters M, Dupont A, Meheus A. Seroprevalence of retroviral infection in women with different fertility statuses in Gabon, western equatorial Africa. J Acquir Immune Defic Syndr. 1991;4:468–70.
Mvé MT, Bisvigou U, Barry NC, Ondo CE, Nkoghe D. Reasons for stopping and restarting tuberculosis treatment in Libreville (Gabon). Sante. 2010;20:31–4.
Nkoghe D, Toung Mve M, Nnegue S, Okome Nkoume M, Iba BJ, Hypolite J, et al. HIV seroprevalence among tuberculosis patients in Nkembo Hospital, Libreville, Gabon. Short note. Bull Soc Pathol Exot. 2005;98:121–2.
Mouba JF, Miloundja J, Mimbila-Mayi M, Ndjenkam FT, N’zouba L. Cervical lymph node tuberculosis in Libreville: epidemiology, diagnosis, and therapy. Sante. 2011;21:165–8.
Kombila DU, Moussavou-Kombila JB, Grobusch MP, Lell B. Clinical and laboratory features of tuberculosis within a hospital population in Libreville, Gabon. Infection. 2013;41:737–9.
Mbopi-Kéou FX, Dempouo Djomassi L, Monebenimp F. Study of factors related to adherence to antiretroviral therapy among patients followed at HIV/AIDS Unit in the District Hospital of Dschang, Cameroon. Pan Afr Med J. 2012;12:55.
Ebana Mvogo C, Ellong A, Bella AL, Luma H, Achu Joko H. Ocular complications of HIV/AIDS in Cameroon: is there is any correlation with the level of CD4 lymphocytes count? Bull Soc Belge Ophtalmol. 2007;305:7–12.
Ibara JR, Itoua C, Gathse A, Obengui, Gassaye D, Nkoua JL, et al. Acquired immunodeficiency syndrome in elderly persons in a tropical zone. Apropos of 175 cases in the Congo. Bull Soc Pathol Exot. 2002;95:100–2.
Okome Nkoumou MM, Okome Essima R, Obiang Ndong GP, Okome Miame F. Clinical and laboratory findings in HIV-infected patients at the Jeanne Ebori Foundation in Libreville, Gabon (2002–2005). Med Trop (Mars). 2007;67:357–62.
Kuaban C, Fotsin JG, Koulla-Shiro S, Ekono MR, Hagbe P. Lower lung field tuberculosis in Yaounde, Cameroon. Cent Afr J Med. 1996;42:62–5.
Kuaban C, Koulla-Shiro S, Lekama Assiene T, Hagbe P. Tuberculosis screening of patient contacts in 1993 and 1994 in Yaounde, Cameroon. Med Trop (Mars). 1996;56:156–8.
Pefura Yone EW, Kuaban C, Simo L. Tuberculous pleural effusion in Yaounde, Cameroon: The influence of HIV infection. Rev Mal Respir. 2011;28:1138–45.
Sume GE, Hoshen M, Bita G, Kabore S, Nzima VN. Treatment outcome of TB/HIV positive and negative smear positive pulmonary tuberculosis patients treated using daily self-administered therapy in a Cameroonian district hospital. East Afr Med J. 2009;86:469–75.
Kuaban C, Bame R, Mouangue L, Djella S, Yomgni C. Non conversion of sputum smears in new smear positive pulmonary tuberculosis patients in Yaounde, Cameroon. East Afr Med J. 2009;86:219–25.
Breton G, Service YB, Kassa-Kelembho E, Mbolidi CD, Minssart P. Tuberculosis and HIV in Bangui, Central African Republic: strong prevalence and management difficulties. Med Trop (Mars). 2002;62:623–6.
Chartier L, Leng C, Sire JM, Le Minor O, Saman M, Bercion R, et al. Factors associated with negative direct sputum examination in Asian and African HIV-infected patients with tuberculosis (ANRS 1260). PLoS One. 2011;6:e21212.
Ondounda M, Ilozue C, Mounguengui D, Magne C, Nzenze JR. Clinical and radiological features of tuberculosis during HIV infection in Libreville, Gabon. Med Trop (Mars). 2011;71:253–6.
Engohan Alloghe E, Toung Mve M, Ramarojoana S, Iba J, Nkoghe D. Epidemiology of childhood tuberculosis in Libreville, Gabon from 1997 to 2001. Med Trop (Mars). 2006;66:469–71.
Cuadros DF, Branscum AJ, García-Ramos G. No evidence of association between HIV-1 and malaria in populations with low HIV-1 prevalence. PLoS One. 2011;6:23458.
Roka M, Goñi P, Rubio E, Clavel A. Prevalence of intestinal parasites in HIV-positive patients on the island of Bioko, Equatorial Guinea: its relation to sanitary conditions and socioeconomic factors. Sci Total Environ. 2012;432:404–11.
Mbopi-Kéou FX, Grésenguet G, Mayaud P, Weiss HA, Gopal R, Matta M, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–6.
Mbopi-Kéou FX, Legoff J, Grésenguet G, Si-Mohamed A, Matta M, Mayaud P, et al. Genital shedding of herpes simplex virus-2 DNA and HIV-1 RNA and proviral DNA in HIV-1- and herpes simplex virus-2-coinfected African women. J Acquir Immune Defic Syndr. 2003;33:121–4.
Békondi C, Bernede C, Passone N, Minssart P, Kamalo C, Mbolidi D, et al. Primary and opportunistic pathogens associated with meningitis in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Int J Infect Dis. 2006;10:387–95.
Modia O’Yandjo A, Foidart JM, Rigo J. Influence of HIV-1 and placental malaria co-infection on newborn biometry and Apgar scores in Kinshasa, Democratic Republic of Congo. J Gynecol Obstet Biol Reprod (Paris). 2011;40:460–4.
Perret JL, Moussavou-Kombila JB, Delaporte E, Minko-Mi-Etoua D, Pemba LF, Boguikouma JB, et al. Prevalence of hepatitis B and C virus, HTLV-1 and HIV in type B lymphoproliferative syndromes in Gabon. Bull Soc Pathol Exot. 2003;96:275–8.
World Health Organization (WHO). Progress report 2011: Global HIV/AIDS response. 2011. http://www.who.int/hiv/pub/progress_report2011/en/.
Jao J, Palmer D, Leus I, Tih P, Baweja M, Klotman M, et al. Prevalence and predictors of proteinuria in HIV-infected and uninfected pregnant women in Cameroon. Nephrol Dial Transplant. 2011;26:3051–3.
Nkenfou CN, Lobé EE, Ouwe-Missi-Oukem-Boyer O, Sosso MS, Dambaya B, Gwom LC, et al. Implementation of HIV early infant diagnosis and HIV type 1 RNA viral load determination on dried blood spots in Cameroon: challenges and propositions. AIDS Res Hum Retroviruses. 2012;28:176–81.
Torpey K, Kasonde P, Kabaso M, Weaver MA, Bryan G, Mukonka V, et al. Reducing pediatric HIV infection: estimating mother-to-child transmission rates in a program setting in Zambia. J Acquir Immune Defic Syndr. 2010;54:415–22.
Kouanda S, Tougri H, Cisse M, Simpore J, Pietra V, Doulougou B, et al. Impact of maternal HAART on the prevention of mother-to-child transmission of HIV: results of an 18-month follow-up study in Ouagadougou, Burkina Faso. AIDS Care. 2010;22:843–50.
Namukwaya Z, Mudiope P, Kekitiinwa A, Musoke P, Matovu J, Kayma S, et al. The impact of maternal highly active antiretroviral therapy and short-course combination antiretrovirals for prevention of mother-to-child transmission on early infant infection rates at the Mulago national referral hospital in Kampala, Uganda, January 2007 to May 2009. J Acquir Immune Defic Syndr. 2011;56:69–75.
Azcoaga-Lorenzo A, Ferreyra C, Alvarez A, Palma PP, Velilla E, del Amo J. Effectiveness of a PMTCT programme in rural Western Kenya. AIDS Care. 2011;23:274–80.
Fitzgerald FC, Bekker LG, Kaplan R, Myer L, Lawn SD, Wood R. Mother-to-child transmission of HIV in a community-based antiretroviral clinic in South Africa. S Afr Med J. 2010;100:827–31.
Mirkuzie AH, Hinderaker SG, Sisay MM, Moland KM, Mørkve O. Current status of medication adherence and infant follow up in the prevention of mother to child HIV transmission programme in Addis Ababa: a cohort study. J Int AIDS Soc. 2011;14:50.
Ikechebelu JI, Ugboaja JO, Kalu SO, Ugochukwu EF. The outcome of prevention of mother to child transmission (PMTCT) of HIV infection programme in Nnewi, southeast Nigeria. Niger J Med. 2011;20:421–5.
Kouanfack C, Aghokeng AF, Mondain AM, Bourgeois A, Kenfack A, Mpoudi-Ngolé E, et al. Lamivudine-resistant HBV infection in HIV-positive patients receiving antiretroviral therapy in a public routine clinic in Cameroon. Antivir Ther. 2012;17:321–6.
Laurent C, Kouanfack C, Laborde-Balen G, Aghokeng AF, Mbougua JB, Boyer S, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessments versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratall ANRS 12110/ESTHER): a randomised non-inferiority trial. Lancet Infect Dis. 2011;11:825–33.
Van Rompaey S, Kimfuta J, Kimbondo P, Monn C, Buvé A. Operational assessment of access to ART in rural Africa: the example of Kisantu in Democratic Republic of the Congo. AIDS Care. 2011;23:686–93.
Ryder RW, Kamenga C, Jingu M, Mbuyi N, Mbu L, Behets F. Pregnancy and HIV-1 incidence in 178 married couples with discordant HIV-1 serostatus: additional experience at an HIV-1 counselling centre in the Democratic Republic of the Congo. Trop Med Int Health. 2000;5:482–7.
Laurent C, Meilo H, Guiard-Schmid JB, Mapouré Y, Noël JM, M’Bangué M, et al. Antiretroviral therapy in public and private routine health care clinics in Cameroon: lessons from the Douala antiretroviral (DARVIR) initiative. Clin Infect Dis. 2005;41:108–11.
Marcellin F, Boyer S, Protopopescu C, Dia A, Ongolo-Zogo P, Koulla-Shiro S, et al. Determinants of unplanned antiretroviral treatment interruptions among people living with HIV in Yaoundé, Cameroon (EVAL survey, ANRS 12-116). Trop Med Int Health. 2008;13:1470–8.
Billong SC, Fokam J, Nkwescheu AS, Kembou E, Milenge P, Tsomo Z, et al. Early warning indicators for HIV drug resistance in Cameroon during the year 2010. PLoS One. 2012;7:e36777.
Ndziessi G, Boyer S, Kouanfack C, Cohen J, Marcellin F, Moatti JP, et al. Adherence as a predictor of sexual behaviors in people living with HIV/AIDS during the first year of antiretroviral therapy in rural Cameroon: data from Stratall ANRS 12110/ESTHER trial. PLoS One. 2012;7:e36118.
Muko KN, Ngwa VC, Chigang LC, Ngwa IG, Meiburg A, Shu EN. Willingness to pay for treatment with highly active antiretroviral (HAART) drugs: a rural case study in Cameroon. SAHAR J. 2004;1:107–13.
Yumo HA, Mbanya D, Kuaban C, Neuhann F. Outcome assessment of a global fund grant for tuberculosis control at the district level in rural Cameroon. Int J Tuberc Lung Dis. 2011;15:352–7.
Njozing BN, Edin KE, San Sebastián M, Hurtig AK. Voices from the frontline: counsellors’ perspectives on TB/HIV collaborative activities in the Northwest Region, Cameroon. BMC Health Serv Res. 2011;11:328.
Kuaban C, Bercion R, Jifon G, Cunin P, Blackett KN. Acquired anti-tuberculosis drug resistance in Yaounde, Cameroon. Int J Tuberc Lung Dis. 2000;4:427–32.
Mauclère P, Loussert-Ajaka I, Damond F, Fagot P, Souquières S, Monny Lobe M, et al. Serological and virological characterization of HIV-1 group O infection in Cameroon. AIDS. 1997;11:445–53.
Yamaguchi J, McArthur CP, Vallari A, Coffey R, Bodelle P, Beyeme M, et al. HIV-1 Group N: evidence of ongoing transmission in Cameroon. AIDS Res Hum Retroviruses. 2006;22:453–7.
Brennan CA, Bodelle P, Coffey R, Devare SG, Golden A, Hackett J Jr, et al. The prevalence of diverse HIV-1 strains was stable in Cameroonian blood donors from 1996 to 2004. J Acquir Immune Defic Syndr. 2008;49:432–9.
Burda ST, Konings FA, Williams CA, Anyangwe C, Nyambi PN. HIV-1 CRF09_cpx circulates in the North West Province of Cameroon where CRF02_AG infections predominate and recombinant strains are common. AIDS Res Hum Retroviruses. 2004;20:1358–63.
Carr JK, Wolfe ND, Torimiro JN, Tamoufe U, Mpoudi-Ngole E, Eyzaguirre L, et al. HIV-1 recombinants with multiple parental strains in low-prevalence, remote regions of Cameroon: evolutionary relics? Retrovirology. 2010;7:39.
Konings FA, Haman GR, Xue Y, Urbanski MM, Hertzmark K, Nanfack A, et al. Genetic analysis of HIV-1 strains in rural eastern Cameroon indicates the evolution of second-generation recombinants to circulating recombinant forms. J Acquir Immune Defic Syndr. 2006;42:331–41.
Mboudjeka I, Bikandou B, Zekeng L, Takehisa J, Harada Y, Yamaguchi-Kabata Y, et al. Genetic diversity of HIV-1 group M from Cameroon and Republic of Congo. Arch Virol. 1999;144:2291–311.
Ndembi N, Takehisa J, Zekeng L, Kobayashi E, Ngansop C, Songok EM, et al. Genetic diversity of HIV type 1 in rural eastern Cameroon. J Acquir Immune Defic Syndr. 2004;37:1641–50.
Ndembi N, Iwamoto S, Ngansop C, Lemey P, Abimiku A, Mbanya D, et al. High frequency of HIV-1 dual infections in Cameroon, West Central Africa. J Acquir Immune Defic Syndr. 2011;57:e25–7.
Soares EA, Makamche MF, Siqueira JD, Lumngwena E, Mbuagbaw J, Kaptue L, et al. Molecular diversity and polymerase gene genotypes of HIV-1 among treatment-naïve Cameroonian subjects with advanced disease. J Clin Virol. 2010;48:173–9.
Yamaguchi J, Bodelle P, Vallari AS, Coffey R, McArthur CP, Schochetman G, et al. HIV infections in northwestern Cameroon: identification of HIV type 1 group O and dual HIV type 1 group M and group O infections. AIDS Res Hum Retroviruses. 2004;20:944–57.
Véras NM, Santoro MM, Gray RR, Tatem AJ, Lo Presti A, Olearo F, et al. Molecular epidemiology of HIV type 1 CRF02_AG in Cameroon and African patients living in Italy. AIDS Res Hum Retroviruses. 2011;27:1173–82.
Faria NR, Suchard MA, Abecasis A, Sousa JD, Ndembi N, Bonfim I, et al. Phylodynamics of the HIV-1 CRF02_AG clade in Cameroon. Infect Genet Evol. 2012;12:453–60.
Ortiz M, Sanchez I, Gonzalez MP, León MI, Abeso N, Asumu E, et al. Molecular epidemiology of HIV type 1 subtypes in equatorial guinea. AIDS Res Hum Retroviruses. 2001;17:851–5.
Koyalta D, Charpentier C, Beassamda J, Rey E, Si-Mohamed A, Djemadji-Oudjeil N, et al. High frequency of antiretroviral drug resistance among HIV-infected adults receiving first-line highly active antiretroviral therapy in N’Djamena, Chad. Clin Infect Dis. 2009;49:155–9.
Vidal N, Koyalta D, Richard V, Lechiche C, Ndinaromtan T, Djimasngar A, et al. High genetic diversity of HIV-1 strains in Chad, West Central Africa. J Acquir Immune Defic Syndr. 2003;33:239–46.
Djoko CF, Rimoin AW, Vidal N, Tamoufe U, Wolfe ND, Butel C, et al. High HIV type 1 group M pol diversity and low rate of antiretroviral resistance mutations among the uniformed services in Kinshasa, Democratic Republic of the Congo. AIDS Res Hum Retroviruses. 2011;27:323–9.
Mokili JL, Wade CM, Burns SM, Cutting WA, Bopopi JM, Green SD, et al. Genetic heterogeneity of HIV type 1 subtypes in Kimpese, rural Democratic Republic of Congo. AIDS Res Hum Retroviruses. 1999;15:655–64.
Vidal N, Peeters M, Mulanga-Kabeya C, Nzilambi N, Robertson D, Ilunga W, et al. Unprecedented degree of human immunodeficiency virus type 1 (HIV-1) group M genetic diversity in the Democratic Republic of Congo suggests that the HIV-1 pandemic originated in Central Africa. J Virol. 2000;74:10498–507.
Bikandou B, Takehisa J, Mboudjeka I, Ido E, Kuwata T, Miyazaki Y, et al. Genetic subtypes of HIV type 1 in Republic of Congo. AIDS Res Hum Retroviruses. 2000;16:613–9.
Candotti D, Tareau C, Barin F, Joberty C, Rosenheim M, M’Pele P, et al. Genetic subtyping and V3 serotyping of HIV type 1 isolates in Congo. AIDS Res Hum Retroviruses. 1999;15:309–14.
Niama FR, Toure-Kane C, Vidal N, Obengui P, Bikandou B, Ndoundou Nkodia MY, et al. HIV-1 subtypes and recombinants in the Republic of Congo. Infect Genet Evol. 2006;6:337–43.
Caron M, Makuwa M, Souquière S, Descamps D, Brun-Vézinet F, Kazanji M. Human immunodeficiency virus type 1 seroprevalence and antiretroviral drug resistance-associated mutations in miners in Gabon, central Africa. AIDS Res Hum Retroviruses. 2008;24:1225–8.
Mintsa-Ndong A, Caron M, Plantier JC, Makuwa M, Le Hello S, Courgnaud V, et al. High HIV Type 1 prevalence and wide genetic diversity with dominance of recombinant strains but low level of antiretroviral drug-resistance mutations in untreated patients in northeast Gabon, Central Africa. AIDS Res Hum Retroviruses. 2009;25:411–8.
Burda ST, Viswanath R, Zhao J, Kinge T, Anyangwe C, Tinyami ET, et al. HIV-1 reverse transcriptase drug-resistance mutations in chronically infected individuals receiving or naïve to HAART in Cameroon. J Med Virol. 2010;82:187–96.
Charpentier C, Gody JC, Tisserand P, Matta M, Fournier J, Mbitikon O, et al. Usefulness of a genotypic resistance test using dried blood spot specimens in African HIV-infected children with virological failure according to the 2010-revised WHO criteria. Arch Virol. 2011;156:1603–6.
Ragupathy V, Zhao J, Wood O, Tang S, Lee S, Nyambi P, et al. Identification of new, emerging HIV-1 unique recombinant forms and drug resistant viruses circulating in Cameroon. Virol J. 2011;8:185.
Ceccarelli L, Salpini R, Moudourou S, Cento V, Santoro MM, Fokam J, et al. Characterization of drug resistance mutations in naive and ART-treated patients infected with HIV-1 in Yaounde, Cameroon. J Med Virol. 2012;84:721–7.
Fokam J, Salpini R, Santoro MM, Cento V, Perno CF, Colizzi V, et al. Drug resistance among drug-naive and first-line antiretroviral treatment-failing children in Cameroon. Pediatr Infect Dis J. 2011;30:1062–8.
Péré H, Charpentier C, Mbelesso P, Dandy M, Matta M, Moussa S, et al. Virological response and resistance profiles after 24 months of first-line antiretroviral treatment in adults living in Bangui, Central African Republic. AIDS Res Hum Retroviruses. 2012;28:315–23.
Charpentier C, Gody JC, Tisserand P, Matta M, Péré H, Fournier J, et al. Surveillance of antiretroviral drug resistance mutations in untreated young children living in the Central African Republic. Antivir Ther. 2011;16:1347–50.
Charpentier C, Gody JC, Mbitikon O, Moussa S, Matta M, Péré H, et al. Virological response and resistance profiles after 18 to 30 months of first- or second-/third-line antiretroviral treatment: a cross-sectional evaluation in HIV type 1-infected children living in the Central African Republic. AIDS Res Hum Retroviruses. 2012;28:87–94.
Vergne L, Malonga-Mouellet G, Mistoul I, Mavoungou R, Mansaray H, Peeters M, et al. Resistance to antiretroviral treatment in Gabon: need for implementation of guidelines on antiretroviral therapy use and HIV-1 drug resistance monitoring in developing countries. J Acquir Immune Defic Syndr. 2002;1:165–8.
Assam-Assam JP, Penlap VB, Cho-Ngwa F, Tedom JC, Ane-Anyangwe I, Titanji VP. Mycobacterium tuberculosis complex drug resistance pattern and identification of species causing tuberculosis in the West and Centre regions of Cameroon. BMC Infect Dis. 2011;11:94.
Bercion R, Kuaban C. Initial resistance to antituberculosis drugs in Yaounde, Cameroon in 1995. Int J Tuberc Lung Dis. 1997;1:110–4.
Kassa-Kelembho E, Bobossi-Serengbe G, Takeng EC, Nambea-Koisse TB, Yapou F, Talarmin A. Surveillance of drug-resistant childhood tuberculosis in Bangui, Central African Republic. Int J Tuberc Lung Dis. 2004;8:574–8.
Diguimbaye C, Hilty M, Ngandolo R, Mahamat HH, Pfyffer GE, Baggi F, et al. Molecular characterization and drug resistance testing of Mycobacterium tuberculosis isolates from Chad. J Clin Microbiol. 2006;44:1575–7.
Abdelhadi O, Ndokaïn J, Ali MM, Friocourt V, Mortier E, Heym B. Drug resistance testing of Mycobacterium tuberculosis isolates from sputum in Chad. Bull Soc Pathol Exot. 2012;105:16–22.
Minime-Lingoupou F, Pierre-Audigier C, Kassa-Kélémbho E, Barilone N, Zandanga G, Rauzier J, et al. Rapid identification of multidrug-resistant tuberculosis isolates in treatment failure or relapse patients in Bangui, Central African Republic. Int J Tuberc Lung Dis. 2010;14:782–5.
Kabedi MJ, Kashongwe M, Kayembe JM, Mumba Ngoyi D, Mampasi P, Mbaya P, et al. Primary resistance of Mycobacterium tuberculosis to anti-tuberculosis drugs in Kinshasa, (DRC). Bull Soc Pathol Exot. 2007;100:275–6.
Mounguengui D, Ondounda M, Mandji Lawson JM, Fabre M, Gaudong L, Mangouka L, et al. Multi-resistant tuberculosis at the hôpital d’instruction des armées de Libreville (Gabon) about 16 cases. Bull Soc Pathol Exot. 2012;105:1–4.
Conflict of interest
None of the authors has any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janssen, S., Huson, M.A.M., Bélard, S. et al. TB and HIV in the Central African region: current knowledge and knowledge gaps. Infection 42, 281–294 (2014). https://doi.org/10.1007/s15010-013-0568-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-013-0568-y