Abstract
Background
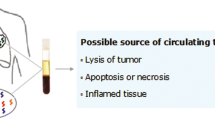
The release of DNA into peripheral blood is a common event in cancer patients, occurring as a consequence of necrotic and apoptotic processes typical of tumor cells. However, free circulating DNA (fcDNA) is also present in patients with benign diseases and in healthy individuals. Both quantitative and qualitative aspects of fcDNA have been studied as potential biomarkers in a number of tumor types. In particular, quantitative analysis of fcDNA has been shown to play an important role in the diagnosis of non-small cell lung cancer (NSCLC), because of its ability to discriminate between healthy subjects and individuals with NSCLC. Additionally, fcDNA in cancer patients derives predominantly from tumor tissue and, as such, it can be used for the molecular characterization of the primary tumor. Targeted therapies in NSCLC have, in recent years, produced promising results, highlighting the importance of molecular profiling of the primary cancer lesions. Considering that little or no tumor material is available for at least some of the patients, the possibility of using fcDNA for molecular analysis becomes increasingly important. In the present review we evaluated several quantitative and qualitative aspects of fcDNA that could be instrumental for the differential diagnosis of lung disease.
Conclusions
There is ample evidence in the literature to support the possible use of peripheral blood-derived fcDNA in the early diagnosis and molecular characterization of lung cancer. This non-invasive method may also turn out to be valuable in monitoring drug response and in identifying induced mechanisms of drug resistance. Before it can be implemented in routine clinical practice, however, additional efforts are needed to standardize the methodology.
Similar content being viewed by others
References
R. Swaminathan, A.N. Butt, Circulating nucleic acids in plasma and serum: recent developments. Ann. N. Y. Acad. Sci. 1075, 1–9 (2006)
K. Jung, M. Fleischhacker, A. Rabien, Cell-free DNA in the blood as a solid tumor biomarker- a critical appraisal of the literatue. Clin. Chim. Acta 411(21–22), 1611–1624 (2010)
A. Ziegler, U. Zangemeister-Wittke, R.A. Stahel, Circulating DNA: a new diagnostic gold mine? Cancer Treat. Rev. 28(5), 255–271 (2002)
N. Suzuki, A. Kamataki, J. Yamaki, Y. Homma, Characterization of circulating DNA in healthy human plasma. Clin. Chim. Acta 387(1–2), 55–58 (2008)
M. Stroun, P. Maurice, V. Vasioukhin, J. Lyautey, C. Lederrey, F. Lefort, A. Rossier, X.Q. Chen, P. Anker, The origin and mechanism of circulating DNA. Ann. N. Y. Acad. Sci. 906, 161–168 (2000)
M. Stroun, J. Lyautey, C. Lederrey, A. Olson-Sand, P. Anker, About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta 313(1–2), 139–142 (2001)
C.N. Li, H.L. Hsu, T.L. Wu, K.C. Tsao, C.F. Sun, J.T. Wu, Cell-free DNA is released from tumor cells upon cell death: a study of tissue cultures of tumor cell lines. J. Clin. Lab. Anal. 17(4), 103–107 (2003)
D.C. García-Olmo, J. Samos, M.G. Picazo, A.I. Asensio, I. Toboso, D. García-Olmo, Release of cell-free DNA into the bloodstream leads to high levels of non-tumor plasma DNA during tumor progression in rats. Cancer Lett. 272(1), 133–140 (2008)
H. Fiegl, S. Millinger, E. Mueller-Holzner, C. Marth, C. Ensinger, A. Berger, H. Klocker, G. Goebel, M. Widschwendter, Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 65(4), 1141–1145 (2005)
A. Altimari, A.D. Grigioni, E. Benedettini, E. Gabusi, R. Schiavina, A. Martinelli, A.M. Morselli-Labate, G. Martorana, W.F. Grigioni, M. Fiorentino, Diagnostic role of circulating free plasma DNA detection in patients with localized prostate cancer. Am. J. Clin. Pathol. 129(5), 756–762 (2008)
P.J. Bastian, G.S. Palapattu, S. Yegnasubramanian, X. Lin, C.G. Rogers, L.A. Mangold, B. Trock, M. Eisenberger, A.W. Partin, W.G. Nelson, Prognostic value of preoperative serum cell-free circulating DNA in men with prostate cancer undergoing radical prostatectomy. Clin. Cancer Res. 13(18 Pt 1), 5361–5367 (2007)
E. Papadopoulou, E. Davilas, V. Sotiriou, E. Georgakopoulos, S. Georgakopoulou, A. Koliopanos, F. Aggelakis, K. Dardoufas, N.J. Agnanti, I. Karydas, G. Nasioulas, Cell-free DNA and RNA in plasma as a new molecular marker for prostate and breast cancer. Ann. N. Y. Acad. Sci. 1075, 235–243 (2006)
K. Jung, C. Stephan, M. Lewandowski, S. Klotzek, M. Jung, G. Kristiansen, M. Lein, S.A. Loening, D. Schnorr, Increased cell-free DNA in plasma of patients with metastatic spread in prostate cancer. Cancer Lett. 205(2), 173–180 (2004)
X.Y. Zhong, A. Ladewig, S. Schmid, E. Wight, S. Hahn, W. Holzgreve, Elevated level of cell-free plasma DNA is associated with breast cancer. Arch. Gynecol. Obstet. 276(4), 327–331 (2007)
C. Kohler, R. Radpour, Z. Barekati, R. Asadollahi, J. Bitzer, E. Wight, N. Bürki, C. Diesch, W. Holzgreve, X.Y. Zhong, Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Mol. Cancer 8, 105 (2009)
H. Schwarzenbach, V. Müller, N. Stahmann, K. Pantel, Detection and characterization of circulating microsatellite-DNA in blood of patients with breast cancer. Ann. N. Y. Acad. Sci. 1022, 25–32 (2004)
G. Sozzi, D. Conte, M. Leon, R. Ciricione, L. Roz, C. Ratcliffe, E. Roz, N. Cirenei, M. Bellomi, G. Pelosi, M.A. Pierotti, U. Pastorino, Quantification of free circulating DNA as a diagnostic marker in lung cancer. J. Clin. Oncol. 21(21), 3902–3908 (2003)
P. Ulivi, L. Mercatali, W. Zoli, D. Dell’amore, V. Poletti, G.L. Casoni, E. Scarpi, E. Flamini, D. Amadori, R. Silvestrini, Serum free DNA and COX-2 mRNA expression in peripheral blood for lung cancer detection. Thorax 63(9), 843–844 (2008)
E. Flamini, L. Mercatali, O. Nanni, D. Calistri, R. Nunziatini, W. Zoli, P. Rosetti, N. Gardini, A. Lattuneddu, G.M. Verdecchia, D. Amadori, Free DNA and carcinoembryonic antigen serum levels: an important combination for diagnosis of colorectal cancer. Clin. Cancer Res. 12(23), 6985–6988 (2006)
R. Catarino, M.M. Ferreira, H. Rodrigues, A. Coelho, A. Nogal, A. Sousa, R. Medeiros, Quantification of free circulating tumor DNA as a diagnostic marker for breast cancer. DNA Cell Biol. 27(8), 415–421 (2008)
C.P. Chang, R.H. Chia, T.L. Wu, K.C. Tsao, C.F. Sun, J.T. Wu, Elevated cell-free serum DNA detected in patients with myocardial infarction. Clin. Chim. Acta 327(1–2), 95–101 (2003)
G.J. Fournié, F. Martres, J.P. Pourrat, C. Alary, M. Rumeau, Plasma DNA as cell death marker in elderly patients. Gerontology 39(4), 215–221 (1993)
T.H. Rainer, N.Y. Lam, Circulating nucleic acids and critical illness. Ann. N. Y. Acad. Sci. 1075, 271–277 (2006)
J.M. Silva, J. Silva, A. Sanchez, J.M. Garcia, G. Dominguez, M. Provencio, L. Sanfrutos, E. Jareño, A. Colas, P. España, F. Bonilla, Tumor DNA in plasma at diagnosis of breast cancer patients is a valuable predictor of disease-free survival. Clin. Cancer Res. 8(12), 3761–3766 (2002)
N. Ren, Q.H. Ye, L.X. Qin, B.H. Zhang, Y.K. Liu, Z.Y. Tang, Circulating DNA level is negatively associated with the long-term survival of hepatocellular carcinoma patients. World J. Gastroenterol. 12(24), 3911–3914 (2006)
O. Gautschi, C. Bigosch, B. Huegli, M. Jermann, A. Marx, E. Chassé, D. Ratschiller, W. Weder, M. Joerger, D.C. Betticher, R.A. Stahel, A. Ziegler, Circulating deoxyribonucleic Acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J. Clin. Oncol. 22(20), 4157–4164 (2004)
A.A. Kamat, M. Baldwin, D. Urbauer, D. Dang, L.Y. Han, A. Godwin, B.Y. Karlan, J.L. Simpson, D.M. Gershenson, R.L. Coleman, F.Z. Bischoff, A.K. Sood, Plasma cell-free DNA in ovarian cancer: an independent prognostic biomarker. Cancer 116(8), 1918–1925 (2010)
Z.H. Huang, L.H. Li, D. Hua, Quantitative analysis of plasma circulating DNA at diagnosis and during follow-up of breast cancer patients. Cancer Lett. 243(1), 64–70 (2006)
M. Frattini, G. Gallino, S. Signoroni, D. Balestra, L. Lusa, L. Battaglia, G. Sozzi, L. Bertario, E. Leo, S. Pilotti, M.A. Pierotti, Quantitative and qualitative characterization of plasma DNA identifies primary and recurrent colorectal cancer. Cancer Lett. 263(2), 170–181 (2008)
F. Banki, R.J. Mason, D. Oh, J.A. Hagen, S.R. DeMeester, J.C. Lipham, K. Tanaka, K.D. Danenberg, W.N. Yacoub, P.V. Danenberg, T.R. DeMeester, Plasma DNA as a molecular marker for completeness of resection and recurrent disease in patients with esophageal cancer. Arch. Surg. 142(6), 533–538 (2007)
R.A. Perego, M. Corizzato, P. Brambilla, S. Ferrero, C. Bianchi, E. Fasoli, S. Signorini, B. Torsello, L. Invernizzi, S. Bombelli, V. Angeloni, M. Pitto, C. Battaglia, V. Proserpio, F. Magni, G. Galasso, P. Mocarelli, Concentration and microsatellite status of plasma DNA for monitoring patients with renal carcinoma. Eur. J. Cancer 44(7), 1039–1047 (2008)
G. Sozzi, D. Conte, L. Mariani, S. Lo Vullo, L. Roz, C. Lombardo, M.A. Pierotti, L. Tavecchio, Analysis of circulating tumor DNA in plasma at diagnosis and during follow-up of lung cancer patients. Cancer Res. 61(12), 4675–4678 (2001)
B.G. Wang, H.Y. Huang, Y.C. Chen, R.E. Bristow, K. Kassauei, C.C. Cheng, R. Roden, L.J. Sokoll, D.W. Chan, S. IeM, Increased plasma DNA integrity in cancer patients. Cancer Res. 63(14), 3966–3968 (2003)
N. Umetani, J. Kim, S. Hiramatsu, H.A. Reber, O.J. Hines, A.J. Bilchik, D.S. Hoon, Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: direct quantitative PCR for ALU repeats. Clin. Chem. 52(6), 1062–1069 (2006)
N. Umetani, A.E. Giuliano, S.H. Hiramatsu, F. Amersi, T. Nakagawa, S. Martino, D.S. Hoon, Prediction of breast tumor progression by integrity of free circulating DNA in serum. J. Clin. Oncol. 24(26), 4270–4276 (2006)
R. Hanley, K.M. Rieger-Christ, D. Canes, N.R. Emara, A.P. Shuber, K.A. Boynton, J.A. Libertino, I.C. Summerhayes, DNA integrity assay: a plasma-based screening tool for the detection of prostate cancer. Clin. Cancer Res. 12(15), 4569–4574 (2006)
H. Tomita, D. Ichikawa, D. Ikoma, S. Sai, N. Tani, H. Ikoma, H. Fujiwara, S. Kikuchi, K. Okamoto, T. Ochiai, E. Otsuji, Quantification of circulating plasma DNA fragments as tumor markers in patients with esophageal cancer. Anticancer Res. 27(4C), 2737–2741 (2007)
W.W. Jiang, M. Zahurak, D. Goldenberg, Y. Milman, H.L. Park, W.H. Westra, W. Koch, D. Sidransky, J. Califano, Increased plasma DNA integrity index in head and neck cancer patients. Int. J. Cancer 119(11), 2673–2676 (2006)
B. Schmidt, S. Weickmann, C. Witt, M. Fleischhacker, Integrity of cell-free plasma DNA in patients with lung cancer and nonmalignant lung disease. Ann. N. Y. Acad. Sci. 1137, 207–213 (2008)
S. Holdenrieder, A. Burges, O. Reich, F.W. Spelsberg, P. Stieber, DNA integrity in plasma and serum of patients with malignant and benign diseases. Ann. N. Y. Acad. Sci. 1137, 162–170 (2008)
E. Gormally, P. Vineis, G. Matullo, F. Veglia, E. Caboux, E. Le Roux, M. Peluso, S. Garte, S. Guarrera, A. Munnia, L. Airoldi, H. Autrup, C. Malaveille, A. Dunning, K. Overvad, A. Tjønneland, E. Lund, F. Clavel-Chapelon, H. Boeing, A. Trichopoulou, D. Palli, V. Krogh, R. Tumino, S. Panico, H.B. Bueno-de-Mesquita, P.H. Peeters, G. Pera, C. Martinez, M. Dorronsoro, A. Barricarte, C. Navarro, J.R. Quirós, G. Hallmans, N.E. Day, T.J. Key, R. Saracci, R. Kaaks, E. Riboli, P. Hainaut, TP53 and KRAS2 mutations in plasma DNA of healthy subjects and subsequent cancer occurrence: a prospective study. Cancer Res. 66(13), 6871–6876 (2006)
Z.M. Shao, J. Wu, Z.Z. Shen, M. Nguyen, p53 mutation in plasma DNA and its prognostic value in breast cancer patients. Clin. Cancer Res. 7(8), 2222–2227 (2001)
T. Ito, K. Kaneko, R. Makino, K. Konishi, T. Kurahashi, H. Ito, A. Katagiri, M. Kushima, M. Kusano, K. Mitamura, M. Imawari, Clinical significance in molecular detection of p53 mutation in serum of patients with colorectal carcinoma. Oncol. Rep. 10(6), 1937–1942 (2003)
T. Kimura, W.S. Holland, T. Kawaguchi, S.K. Williamson, K. Chansky, J.J. Crowley, J.H. Doroshow, H.J. Lenz, D.R. Gandara, P.H. Gumerlock, Mutant DNA in plasma of lung cancer patients: potential for monitoring response to therapy. Ann. N. Y. Acad. Sci. 1022, 55–60 (2004)
J. Däbritz, J. Hänfler, R. Preston, J. Stieler, H. Oettle, Detection of Ki-ras mutations in tissue and plasma samples of patients with pancreatic cancer using PNA-mediated PCR clamping and hybridisation probes. Br. J. Cancer 92(2), 405–412 (2005)
R.E. Board, L. Knight, A. Greystoke, F.H. Blackhall, A. Hughes, C. Dive, M. Ranson, Biomark. Insights 2, 307–319 (2007)
G.S. Xie, A.R. Hou, L.Y. Li, Y.N. Gao, S.J. Cheng, Quantification of plasma DNA as a screening tool for lung cancer. Chin. Med. J. (Engl.) 117(10), 1485–1488 (2004)
L.J. Herrera, S. Raja, W.E. Gooding, T. El-Hefnawy, L. Kelly, J.D. Luketich, T.E. Godfrey, Quantitative analysis of circulating plasma DNA as a tumor marker in thoracic malignancies. Clin. Chem. 51(1), 113–118 (2005)
V. Ludovini, L. Pistola, V. Gregorc, I. Floriani, E. Rulli, S. Piattoni, L. Di Carlo, A. Semeraro, S. Darwish, F.R. Tofanetti, L. Stocchi, Z. Mihaylova, G. Bellezza, R. Del Sordo, G. Daddi, L. Crinò, M. Tonato, Plasma DNA, microsatellite alterations, and p53 tumor mutations are associated with disease-free survival in radically resected non-small cell lung cancer patients: a study of the perugia multidisciplinary team for thoracic oncology. J. Thorac. Oncol. 3(4), 365–373 (2008)
M. Paci, S. Maramotti, E. Bellesia, D. Formisano, L. Albertazzi, T. Ricchetti, G. Ferrari, V. Annessi, D. Lasagni, C. Carbonelli, S. De Franco, M. Brini, G. Sgarbi, R. Lodi, Circulating plasma DNA as diagnostic biomarker in non-small cell lung cancer. Lung Cancer 64(1), 92–97 (2009)
K.A. Yoon, S. Park, S.H. Lee, J.H. Kim, J.S. Lee, Comparison of circulating plasma DNA levels between lung cancer patients and healthy controls. Mol. Diagn. 11(3), 182–185 (2009)
A. Szpechcinski, M. Dancewicz, P. Kopinski, J. Kowalewski, J. Chorostowska-Wynimko, Real-time PCR quantification of plasma DNA in non-small cell lung cancer patients and healthy controls. Eur. J. Med. Res. 14(Suppl 4), 237–240 (2009)
S. Kumar, R. Guleria, V. Singh, A.C. Bharti, A. Mohan, B.C. Das, Efficacy of circulating plasma DNA as a diagnostic tool for advanced non-small cell lung cancer and its predictive utility for survival and response to chemotherapy. Lung Cancer 70(2), 211–217 (2010)
A. Szpechcinski, J. Chorostowska-Wynimko, W. Kupis, K. Maszkowska-Kopij, M. Dancewicz, J. Kowalewski, T. Orlowski, Quantitative analysis of free-circulating DNA in plasma of patients with resectable NSCLC. Expert Opin. Biol. 12(Suppl 1), S3–S9 (2012)
R. Catarino, A. Coelho, A. Araújo, M. Gomes, A. Nogueira, C. Lopes, R. Medeiros, Circulating DNA: diagnostic tool and predictive marker for overall survival of NSCLC patients. PLoS One 7(6), e38559 (2012)
P. Ulivi, L. Mercatali, G.L. Casoni, E. Scarpi, L. Bucchi, R. Silvestrini, S. Sanna, M. Monteverde, D. Amadori, V. Poletti, W. Zoli, Multiple marker detection in peripheral blood for NSCLC diagnosis. PLoS One 8(2), e57401 (2013)
R. Zhang, F. Shao, X. Wu, K. Ying, Value of quantitative analysis of circulating cell free DNA as a screening tool for lung cancer: a meta-analysis. Lung Cancer 69(2), 225–231 (2010)
R. Rami-Porta, D. Ball, J. Crowley, D.J. Giroux, J. Jett, W.D. Travis, M. Tsuboi, E. Vallières, P. Goldstraw, International Staging Committee; Cancer Research and Biostatistics; Observers to the Committee; Participating Institutions, The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 2(7), 593–602 (2007)
Y. Saito, N. Nagamoto, S. Ota, M. Sato, M. Sagawa, K. Kamma, S. Takahashi, K. Usuda, C. Endo, T. Imai, Results of surgical treatment for roentgenographically occult bronchogenic squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 104(2), 401–407 (1992)
G. Sozzi, L. Roz, D. Conte, L. Mariani, F. Andriani, S. Lo Vullo, C. Verri, U. Pastorino, Plasma DNA quantification in lung cancer computed tomography screening: five-year results of a prospective study. Am. J. Respir. Crit. Care Med. 179(1), 69–74 (2009)
G.L. Casoni, P. Ulivi, L. Mercatali, M. Chilosi, S. Tomassetti, M. Romagnoli, C. Ravaglia, C. Gurioli, C. Gurioli, W. Zoli, R. Silvestrini, V. Poletti, Increased levels of free circulating DNA in patients with idiopathic pulmonary fibrosis. Int. J. Biol. Markers 25(4), 229–235 (2010)
R. Sirera, R.M. Bremnes, A. Cabrera, E. Jantus-Lewintre, E. Sanmartìn, A. Blasco, N. del Pozo, R. Rosell, R. Guijarro, J. Galbis, J.J. Sànchez, C. Camps, Circulating DNA is a useful prognostic factor in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 6(2), 286–290 (2011)
M.A. van der Drift, B.E. Hol, C.H. Klaassen, C.F. Prinsen, Y.A. van Aarssen, R. Donders, J.W. van der Stappen, P.N. Dekhuijzen, H.F. van der Heijden, F.B. Thunnissen, Circulating DNA is a non-invasive prognostic factor for survival in non-small cell lung cancer. Lung Cancer 68(2), 283–287 (2010)
A.K. Pathak, M. Bhutani, S. Kumar, A. Mohan, R. Guleria, Circulating cell-free DNA in plasma/serum of lung cancer patients as a potential screening and prognostic tool. Clin. Chem. 52(10), 1833–1842 (2006)
M. Beau-Faller, M.P. Gaub, A. Schneider, X. Ducrocq, G. Massard, B. Gasser, M.P. Chenard, R. Kessler, P. Anker, M. Strounm, E. Weitzenblum, G. Pauli, J.M. Wihlm, E. Quoix, P. Oudet, Plasma DNA microsatellite panel as sensitive and tumor-specific marker in lung cancer patients. Int. J. Cancer 105(3), 361–370 (2003)
Y.J. Lee, K.A. Yoon, J.Y. Han, H.T. Kim, T. Yun, G.K. Lee, H.Y. Kim, J.S. Lee, Circulating cell-free DNA in plasma of never smokers with advanced lung adenocarcinoma receiving gefitinib or standard chemotherapy as first-line therapy. Clin. Cancer Res. 17(15), 5179–5187 (2011)
M. Esteller, M. Sanchez-Cespedes, R. Rosell, D. Sidransky, S.B. Baylin, J.G. Herman, Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res. 59(1), 67–70 (1999)
A. Bearzatto, D. Conte, M. Frattini, N. Zaffaroni, F. Andriani, D. Balestra, L. Tavecchio, M.G. Daidone, G. Sozzi, p16(INK4A) Hypermethylation detected by fluorescent methylation-specific PCR in plasmas from non-small cell lung cancer. Clin. Cancer Res. 8(12), 3782–3787 (2002)
Y. Liu, Q. An, L. Li, D. Zhang, J. Huang, X. Feng, S. Cheng, Y. Gao, Hypermethylation of p16INK4a in Chinese lung cancer patients: biological and clinical implications. Carcinogenesis 24(12), 1897–1901 (2003)
P. Ulivi, W. Zoli, D. Calistri, F. Fabbri, A. Tesei, M. Rosetti, M. Mengozzi, D. Amadori, p16INK4A and CDH13 hypermethylation in tumor and serum of non-small cell lung cancer patients. J. Cell. Physiol. 206(3), 611–615 (2006)
K. Fujiwara, N. Fujimoto, M. Tabata, K. Nishii, K. Matsuo, K. Hotta, T. Kozuki, M. Aoe, K. Kiura, H. Ueoka, M. Tanimoto, Identification of epigenetic aberrant promoter methylation in serum DNA is useful for early detection of lung cancer. Clin. Cancer Res. 11(3), 1219–1225 (2005)
H.S. Hsu, T.P. Chen, C.H. Hung, C.K. Wen, R.K. Lin, H.C. Lee, Y.C. Wang, Characterization of a multiple epigenetic marker panel for lung cancer detection and risk assessment in plasma. Cancer 110(9), 2019–2026 (2007)
K.L. Ostrow, M.O. Hoque, M. Loyo, M. Brait, A. Greenberg, J.M. Siegfried, J.R. Grandis, A. Gaither Davis, W.L. Bigbee, W. Rom, D. Sidransky, Molecular analysis of plasma DNA for the early detection of lung cancer by quantitative methylation-specific PCR. Clin. Cancer Res. 16(13), 3463–3472 (2010)
Y. Zhang, R. Wang, H. Song, G. Huang, J. Yi, Y. Zheng, J. Wang, L. Chen, Methylation of multiple genes as a candidate biomarker in non-small cell lung cancer. Cancer Lett. 303(1), 21–28 (2011)
S. Begum, M. Brait, S. Dasgupta, K.L. Ostrow, M. Zahurak, A.L. Carvalho, J.A. Califano, S.N. Goodman, W.H. Westra, M.O. Hoque, D. Sidransky, An epigenetic marker panel for detection of lung cancer using cell-free serum DNA. Clin. Cancer Res. 17(13), 4494–4503 (2011)
K. Woodson, J. Mason, S.W. Choi, T. Hartman, J. Tangrea, J. Virtamo, P.R. Taylor, D. Albanes, Hypomethylation of p53 in peripheral blood DNA is associated with the development of lung cancer. Cancer Epidemiol. Biomarkers Prev. 10(1), 69–74 (2001)
T.S. Mok, Y.L. Wu, S. Thongprasert, C.H. Yang, D.T. Chu, N. Saijo, P. Sunpaweravong, B. Han, B. Margono, Y. Ichinose, Y. Nishiwaki, Y. Ohe, J.J. Yang, B. Chewaskulyong, H. Jiang, E.L. Duffield, C.L. Watkins, A.A. Armour, M. Fukuoka, Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361(10), 947–957 (2009)
R. Rosell, E. Carcereny, R. Gervais, A. Vergnenegre, B. Massuti, E. Felip, R. Palmero, R. Garcia-Gomez, C. Pallares, J.M. Sanchez, R. Porta, M. Cobo, P. Garrido, F. Longo, T. Moran, A. Insa, F. De Marinis, R. Corre, I. Bover, A. Illiano, E. Dansin, J. de Castro, M. Milella, N. Reguart, G. Altavilla, U. Jimenez, M. Provencio, M.A. Moreno, J. Terrasa, J. Muñoz-Langa, J. Valdivia, D. Isla, M. Domine, O. Molinier, J. Mazieres, N. Baize, R. Garcia-Campelo, G. Robinet, D. Rodriguez-Abreu, G. Lopez-Vivanco, V. Gebbia, L. Ferrera-Delgado, P. Bombaron, R. Bernabe, A. Bearz, A. Artal, E. Cortesi, C. Rolfo, M. Sanchez-Ronco, A. Drozdowskyj, C. Queralt, I. de Aguirre, J.L. Ramirez, J.J. Sanchez, M.A. Molina, M. Taron, L. Paz-Ares, Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie, Associazione Italiana Oncologia Toracica, Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 13(13), 239–246 (2012)
P. Ulivi, W. Zoli, E. Chiadini, L. Capelli, P. Candoli, D. Calistri, R. Silvestrini, M. Puccetti, EGFR and K-ras mutations in cytologic samples from fine-needle aspirates in NSCLC patients. Eur. Respir. J. 40(1), 267–269 (2012)
J.H. Smouse, E.S. Cibas, P.A. Jänne, V.A. Joshi, K.H. Zou, N.I. Lindeman, EGFR mutations are detected comparably in cytologic and surgical pathology specimens of nonsmall cell lung cancer. Cancer 117(1), 67–72 (2009)
S. Savic, C. Tapia, B. Grilli, A. Rufle, M.P. Bihl, A. de Vito Barascud, M. Herzog, L. Terracciano, F. Baty, L. Bubendorf, Comprehensive epidermal growth factor receptor gene analysis from cytological specimens of non-small-cell lung cancers. Br. J. Cancer 98(1), 154–160 (2008)
S. Maheswaran, L.V. Sequist, S. Nagrath, L. Ulkus, B. Brannigan, C.V. Collura, E. Inserra, S. Diederichs, A.J. Iafrate, D.W. Bell, S. Digumarthy, A. Muzikansky, D. Irimia, J. Settleman, R.G. Tompkins, T.J. Lynch, M. Toner, D.A. Haber, Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359(4), 366–377 (2008)
H. Kimura, M. Suminoe, K. Kasahara, T. Sone, T. Araya, S. Tamori, F. Koizumi, K. Nishio, K. Miyamoto, M. Fujimura, S. Nakao, Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA). Br. J. Cancer 97(6), 778–784 (2007)
T.K. Yung, K.C. Chan, T.S. Mok, J. Tong, K.F. To, Y.M. Lo, Single-molecule detection of epidermal growth factor receptor mutations in plasma by microfluidics digital PCR in non-small cell lung cancer patients. Clin. Cancer Res. 15(6), 2076–2084 (2009)
Y. Kuang, A. Rogers, B.Y. Yeap, L. Wang, M. Makrigiorgos, K. Vetrand, S. Thiede, R.J. Distel, P.A. Jänne, Noninvasive detection of EGFR T790M in gefitinib or erlotinib resistant non-small cell lung cancer. Clin. Cancer Res. 15(8), 2630–2636 (2009)
C. He, M. Liu, C. Zhou, J. Zhang, M. Ouyang, N. Zhong, J. Xu, Detection of epidermal growth factor receptor mutations in plasma by mutant-enriched PCR assay for prediction of the response to gefitinib in patients with non-small-cell lung cancer. Int. J. Cancer 125(10), 2393–2399 (2009)
H. Bai, L. Mao, H.S. Wang, J. Zhao, L. Yang, T.T. An, X. Wang, C.J. Duan, N.M. Wu, Z.Q. Guo, Y.X. Liu, H.N. Liu, Y.Y. Wang, J. Wang, Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages IIIB to IV non-small-cell lung cancer. J. Clin. Oncol. 27(16), 2653–2659 (2009)
P.C. Mack, W.S. Holland, R.A. Burich, R. Sangha, L.J. Solis, Y. Li, L.A. Beckett, P.N. Jr Lara, A.M. Davies, D.R. Gandara, EGFR mutations detected in plasma are associated with patient outcomes in erlotinib plus docetaxel-treated non-small cell lung cancer. J. Thorac. Oncol. 4(12), 1466–1472 (2009)
B. Jiang, F. Liu, L. Yang, W. Zhang, H. Yuan, J. Wang, G. Huang, Serum detection of epidermal growth factor receptor gene mutations using mutant-enriched sequencing in Chinese patients with advanced non-small cell lung cancer. J. Int. Med. Res. 39(4), 1392–1401 (2011)
M. Brevet, M.L. Johnson, C.G. Azzoli, M. Ladanyi, Detection of EGFR mutations in plasma DNA from lung cancer patients by mass spectrometry genotyping is predictive of tumor EGFR status and response to EGFR inhibitors. Lung Cancer 73(1), 96–102 (2011)
K. Taniguchi, J. Uchida, K. Nishino, T. Kumagai, T. Okuyama, J. Okami, M. Higashiyama, K. Kodama, F. Imamura, K. Kato, Quantitative detection of EGFR mutations in circulating tumor DNA derived from lung adenocarcinomas. Clin. Cancer Res. 17(24), 7808–7815 (2011)
Y.M. Chen, W.C. Fan, P.C. Tseng, C.M. Tsai, T.Y. Chou, C.H. Wu, K.T. Chou, Y.C. Lee, R.P. Perng, J. Whang-Peng, Plasma epidermal growth factor receptor mutation analysis and possible clinical applications in pulmonary adenocarcinoma patients treated with erlotinib. Oncol. Lett. 3(3), 713–717 (2012)
T. Nakamura, N. Sueoka-Aragane, K. Iwanaga, A. Sato, K. Komiya, N. Kobayashi, S. Hayashi, T. Hosomi, M. Hirai, E. Sueoka, S. Kimura, Application of a highly sensitive detection system for epidermal growth factor receptor mutations in plasma DNA. J. Thorac. Oncol. 7(9), 1369–1381 (2012)
K. Goto, Y. Ichinose, Y. Ohe, N. Yamamoto, S. Negoro, K. Nishio, Y. Itoh, H. Jiang, E. Duffield, R. McCormack, N. Saijo, T. Mok, M. Fukuoka, Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J. Thorac. Oncol. 7(1), 115–121 (2012)
E.A. Punnoose, S. Atwal, W. Liu, R. Raja, B.M. Fine, B.G. Hughes, R.J. Hicks, G.M. Hampton, L.C. Amler, A. Pirzkall, M.R. Lackner, Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin. Cancer Res. 18(8), 2391–2401 (2012)
X. Zhao, R.B. Han, J. Zhao, J. Wang, F. Yang, W. Zhong, L. Zhang, L.Y. Li, M.Z. Wang, Comparison of Epidermal growth factor receptor mutation statuses in tissue and plasma in stage I-IV Non-small cell lung cancer patients. Respiration 85(2), 119–125 (2013)
S. Wang, T. An, J. Wang, J. Zhao, Z. Wang, M. Zhuo, H. Bai, L. Yang, Y. Zhang, X. Wang, J. Duan, Y. Wang, Q. Guo, M. Wu, Potential clinical significance of a plasma-based KRAS mutation analysis in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 16(4), 1324–1330 (2010)
F. Andriani, D. Conte, T. Mastrangelo, M. Leon, C. Ratcliffe, L. Roz, G. Pelosi, P. Goldstraw, G. Sozzi, U. Pastorino, Detecting lung cancer in plasma with the use of multiple genetic markers. Int. J. Cancer 108(1), 91–96 (2004)
G. Sozzi, K. Musso, C. Ratcliffe, P. Goldstraw, M.A. Pierotti, U. Pastorino, Detection of microsatellite alterations in plasma DNA of non-small cell lung cancer patients: a prospect for early diagnosis. Clin. Cancer Res. 5(10), 2689–2692 (1999)
N. Bruhn, T. Beinert, C. Oehm, B. Jandrig, I. Petersen, X.Q. Chen, K. Possinger, M. Fleischhacker, Detection of microsatellite alterations in the DNA isolated from tumor cells and from plasma DNA of patients with lung cancer. Ann. N. Y. Acad. Sci. 906, 72–82 (2000)
M.A. Thijssen, D.W. Swinkels, T.J. Ruers, J.B. de Kok, Difference between free circulating plasma and serum DNA in patients with colorectal liver metastases. Anticancer Res. 22(1A), 421–425 (2002)
Y.Y. Lui, K.W. Chik, R.W. Chiu, C.Y. Ho, C.W. Lam, Y.M. Lo, Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin. Chem. 48(3), 421–427 (2002)
M. Jung, S. Klotzek, M. Lewandowski, M. Fleischhacker, K. Jung, Changes in concentration of DNA in serum and plasma during storage of blood samples. Clin. Chem. 49(6 Pt 1), 1028–1029 (2003)
T.H. Lee, L. Montalvo, V. Chrebtow, M.P. Busch, Quantitation of genomic DNA in plasma and serum samples: higher concentrations of genomic DNA found in serum than in plasma. Transfusion 41(2), 276–282 (2001)
J. Jen, L. Wu, D. Sidransky, An overview on the isolation and analysis of circulating tumor DNA in plasma and serum. Ann. N. Y. Acad. Sci. 906, 8–12 (2000)
Y.Y. Lui, K.W. Chik, Y.M. Lo, Does centrifugation cause the ex vivo release of DNA from blood cells? Clin. Chem. 48(11), 2074–2076 (2002)
X. Xue, M.D. Teare, I. Holen, Y.M. Zhu, P.J. Woll, Optimizing the yield and utility of circulating cell-free DNA from plasma and serum. Clin. Chim. Acta 404(2), 100–104 (2009)
G. Sozzi, L. Roz, D. Conte, L. Mariani, F. Andriani, P. Verderio, U. Pastorino, Effects of prolonged storage of whole plasma or isolated plasma DNA on the results of circulating DNA quantification assays. J. Natl. Cancer Inst. 97(24), 1848–1850 (2005)
J.B. de Kok, J.C. Hendriks, W.W. van Solinge, H.L. Willems, E.J. Mensink, D.W. Swinkels, Use of real-time quantitative PCR to compare DNA isolation methods. Clin. Chem. 44(10), 2201–2204 (1998)
C. Stemmer, M. Beau-Faller, E. Pencreac’h, E. Guerin, A. Schneider, D. Jaqmin, E. Quoix, M.P. Gaub, P. Oudet, Use of magnetic beads for plasma cell-free DNA extraction: toward automation of plasma DNA analysis for molecular diagnostics. Clin. Chem. 49(11), 1953–1955 (2003)
B. Schmidt, S. Weickmann, C. Witt, M. Fleischhacker, Improved method for isolating cell-free DNA. Clin. Chem. 51(8), 1561–1563 (2005)
C.J. Jorgez, D.D. Dang, J.L. Simpson, D.E. Lewis, F.Z. Bischoff, Quantity versus quality: optimal methods for cell-free DNA isolation from plasma of pregnant women. Genet. Med. 8(10), 615–619 (2006)
S.A. Bustin, V. Benes, J.A. Garson, J. Hellemans, J. Huggett, M. Kubista, R. Mueller, T. Nolan, M.W. Pfaffl, G.L. Shipley, J. Vandesompele, C.T. Wittwer, The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55(4), 611–622 (2009)
J.G. Herman, J.R. Graff, S. Myöhänen, B.D. Nelkin, S.B. Baylin, Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. U. S. A. 93(18), 9821–9826 (1996)
C.A. Eads, K.D. Danenberg, K. Kawakami, L.B. Saltz, C. Blake, D. Shibata, P.V. Danenberg, P.W. Laird, MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res. 28(8), E32 (2000)
S.A. Qureshi, M.U. Bashir, A. Yaqinuddin, Utility of DNA methylation markers for diagnosing cancer. Int. J. Surg. 8(3), 194–198 (2010)
T. Tanaka, Y. Nagai, H. Miyazawa, N. Koyama, S. Matsuoka, A. Sutani, A. Huqun, K. Udagawa, Y. Murayama, M. Nagata, Y. Shimizu, K. Ikebuchi, M. Kanazawa, K. Kobayashi, K. Hagiwara, Reliability of the peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based test for epidermal growth factor receptor mutations integrated into the clinical practice for non-small cell lung cancers. Cancer Sci. 98(2), 246–252 (2007)
H. Kimura, Y. Fujiwara, T. Sone, H. Kunitoh, T. Tamura, K. Kasahara, K. Nishio, High sensitivity detection of epidermal growth factor receptor mutations in the pleural effusion of non-small cell lung cancer patients. Cancer Sci. 97(7), 642–648 (2006)
K.A. Heyries, C. Tropini, M. Vaninsberghe, C. Doolin, O.I. Petriv, A. Singhal, K. Leung, C.B. Hughesman, C.L. Hansen, Megapixel digital PCR. Nat. Methods 8(8), 649–651 (2011)
A. Narayan, N.J. Carriero, S.N. Gettinger, J. Kluytenaar, K.R. Kozak, T.I. Yock, N.E. Muscato, P. Ugarelli, R.H. Decker, A.A. Patel, Ultrasensitive measurement of hotspot mutations in tumor DNA in blood using error-suppressed multiplexed deep sequencing. Cancer Res. 72(14), 3492–3498 (2012)
Acknowledgments
The authors would like to thank Ursula Elbling for editing the manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulivi, P., Silvestrini, R. Role of quantitative and qualitative characteristics of free circulating DNA in the management of patients with non-small cell lung cancer. Cell Oncol. 36, 439–448 (2013). https://doi.org/10.1007/s13402-013-0155-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-013-0155-3