Abstract
Purpose
To assess whether the early metabolic response evaluated by 18F-fluorodeoxy-glucose positron emission combined with computed tomography (FDG PET/CT) predicts the morphological, pathological, and cell-cycle responses to neoadjuvant endocrine therapy of hormone receptor-positive primary breast cancer.
Study design
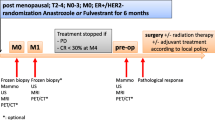
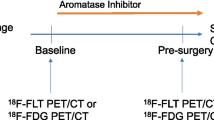
Eleven patients (12 tumors) with estrogen receptor-positive (Allred score 7 or 8) primary breast cancer were enrolled. All patients received a daily dose (2.5 mg) of letrozole for 12 weeks followed by surgery. Sequential FDG PET/CT scans were performed before treatment (baseline), at 4 weeks after the initiation of endocrine therapy (PET2), and prior to surgery (PET3). Tumors showing a 40% or more reduction and those showing a less than 40% reduction in the standardized uptake value maximum (SUVmax) at PET2 compared with the baseline PET were defined as metabolic responders and metabolic nonresponders, respectively. Change in tumor size as measured by ultrasound (morphological response), pathological response, and change in the Ki67 labeling index in tumor tissue (cell-cycle response) during the neoadjuvant letrozole therapy were compared between the metabolic responders and nonresponders.
Results
The average decreases in SUVmax at PET2 compared with the baseline PET in the metabolic responders (n = 6) and the metabolic nonresponders (n = 6) were 60.9% (±21.3 SD) and 14.2% (±12.0 SD), respectively. At PET3 compared with the baseline PET, the metabolic responders showed a significantly higher decrease of 64.5% (±18.7 SD) (p = 0.0004), whereas the nonresponders showed a nonsignificant decrease of 16.7% (±14.1 SD) (p = 0.06). The morphological and pathological responses after letrozole therapy did not differ between the metabolic responders and nonresponders. The metabolic responders showed a marked decrease in the Ki67 labeling index at 2 weeks after the initiation of treatment (62.9%, ±35.9 SD, p = 0.04) and at surgery (91.7%, ±10.7 SD, p = 0.03) compared with the baseline values. In contrast, metabolic nonresponders showed no significant change in the Ki67 index either after 2 weeks of therapy or at surgery.
Conclusion
Cell-cycle response monitored by the Ki67 labeling index correlates with metabolic response monitored by tumor SUVmax. Monitoring of tumor SUVmax using FDG PET/CT may be feasible to predict cell-cycle response to neoadjuvant endocrine therapy of primary breast cancer.





Similar content being viewed by others
References
Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–34.
Macaskill EJ, Renshaw L, Dixon JM. Neoadjuvant use of hormonal therapy in elderly patients with early or locally advanced hormone receptor-positive breast cancer. Oncologist. 2006;11(10):1081–8.
Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat. 2007;105(Suppl 1):33–43.
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–16.
Takei H, Suemasu K, Inoue K, Saito T, Okubo K, Koh J, et al. Multicenter phase II trial of neoadjuvant exemestane for postmenopausal patients with hormone receptor-positive, operable breast cancer: Saitama Breast Cancer Clinical Study Group (SBCCSG-03). Breast Cancer Res Treat. 2008;107(1):87–94.
Ellis MJ. Neoadjuvant endocrine therapy for breast cancer: more questions than answers. J Clin Oncol. 2005;23(22):4842–4.
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, Griffith C, et al. Proliferation and apoptosis as markers of benefit in neoadjuvant endocrine therapy of breast cancer. Clin Cancer Res. 2006;12(3 Pt 2):1024s–30s.
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99(2):167–70.
Ellis MJ, Tao Y, Luo J, A’Hern R, Evans DB, Bhatnagar AS, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100(19):1380–8.
Dehdashti F, Mortimer JE, Trinkaus K, Naughton MJ, Ellis M, Katzenellenbogen JA, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009;113(3):509–17.
Juweid ME, Cheson BD. Positron-emission tomography and assessment of cancer therapy. N Engl J Med. 2006;354(5):496–507.
Weber WA, Ziegler SI, Thodtmann R, Hanauske AR, Schwaiger M. Reproducibility of metabolic measurements in malignant tumors using FDG PET. J Nucl Med. 1999;40(11):1771–7.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38(4):250–8.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35(13):1773–82.
Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47(6):1059–66.
Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–8.
Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24(34):5366–72.
Dose Schwarz J, Bader M, Jenicke L, Hemminger G, Janicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J Nucl Med. 2005;46(7):1144–50.
Ueda S, Kondoh N, Tsuda H, Yamamoto S, Asakawa H, Fukatsu K, et al. Expression of centromere protein F (CENP-F) associated with higher FDG uptake on PET/CT, detected by cDNA microarray, predicts high-risk patients with primary breast cancer. BMC Cancer. 2008;8:384.
Kurosumi M, Akashi-Tanaka S, Akiyama F, Komoike Y, Mukai H, Nakamura S, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version). Breast Cancer. 2008;15(1):5–7.
Tsuda H, Tani Y, Hasegawa T, Fukutomi T. Concordance in judgments among c-erbB-2 (HER2/neu) overexpression detected by two immunohistochemical tests and gene amplification detected by Southern blot hybridization in breast carcinoma. Pathol Int. 2001;51(1):26–32.
Tsuda H, Morita D, Kimura M, Shinto E, Ohtsuka Y, Matsubara O, et al. Correlation of KIT and EGFR overexpression with invasive ductal breast carcinoma of the solid-tubular subtype, nuclear grade 3, and mesenchymal or myoepithelial differentiation. Cancer Sci. 2005;96(1):48–53.
Ueda S, Tsuda H, Sato K, Takeuchi H, Shigekawa T, Matsubara O, et al. Alternative tyrosine phosphorylation of signaling kinases according to hormone receptor status in breast cancer overexpressing the insulin-like growth factor receptor type 1. Cancer Sci. 2006;97(7):597–604.
Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11(2):155–68.
Krainick-Strobel UE, Lichtenegger W, Wallwiener D, Tulusan AH, Janicke F, Bastert G, et al. Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer. 2008;8:62.
Schelling M, Avril N, Nahrig J, Kuhn W, Romer W, Sattler D, et al. Positron emission tomography using [(18)F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18(8):1689–95.
Duch J, Fuster D, Munoz M, Fernandez PL, Paredes P, Fontanillas M, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant chemotherapy in breast cancer. Eur J Nucl Med Mol Imaging. 2009;36:1551–7.
Pons F, Duch J, Fuster D. Breast cancer therapy: the role of PET-CT in decision making. Q J Nucl Med Mol Imaging. 2009;53(2):210–28.
Kurosumi M, Takatsuka Y, Watanabe T, Imoto S, Inaji H, Tsuda H, et al. Histopathological assessment of anastrozole and tamoxifen as preoperative (neoadjuvant) treatment in postmenopausal Japanese women with hormone receptor-positive breast cancer in the PROACT trial. J Cancer Res Clin Oncol. 2008;134(6):715–22.
Tao Y, Klause A, Vickers A, Bae K, Ellis M. Clinical and biomarker endpoint analysis in neoadjuvant endocrine therapy trials. J Steroid Biochem Mol Biol. 2005;95(1–5):91–5.
Ellis MJ, Tao Y, Young O, White S, Proia AD, Murray J, et al. Estrogen-independent proliferation is present in estrogen-receptor HER2-positive primary breast cancer after neoadjuvant letrozole. J Clin Oncol. 2006;24(19):3019–25.
Acknowledgments
This work was supported by grants for the promotion of Defense Medicine from the Ministry of Defense, Japan, and from the Department of Breast Oncology of the International Medical Center at Saitama Medical University. The authors would like to thank Takaaki Suzuki for providing data related to previously published studies.
Conflict of interest statement
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ueda, S., Tsuda, H., Saeki, T. et al. Early metabolic response to neoadjuvant letrozole, measured by FDG PET/CT, is correlated with a decrease in the Ki67 labeling index in patients with hormone receptor-positive primary breast cancer: a pilot study. Breast Cancer 18, 299–308 (2011). https://doi.org/10.1007/s12282-010-0212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-010-0212-y