Summary
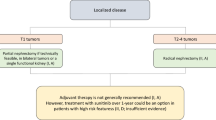
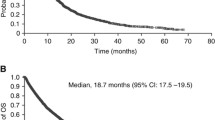
In this short review article we discuss three key oral presentations from the European Society for Medical Oncology (ESMO) Congress 2021 concerning localised, as well as advanced/metastatic renal cell carcinoma, highlighting their potential implications for the improvement of therapeutic modalities in affected patients. (1) Conditional survival and 5‑year follow-up of CheckMate 214 currently represent the longest available phase III follow-up data in the first-line (combination) treatment of clear cell renal cell carcinoma patients with nivolumab + ipilimumab vs. sunitinib. This analysis demonstrated durable efficacy benefits with the respective combination vs. sunitinib. Moreover, conditional survival results predict an increased probability of durable overall survival, progression-free survival, and response rates with nivolumab + ipilimumab at 2‑ and 3‑year landmarks. (2) The randomised, double-blind, phase III KEYNOTE-564 study, presented as a highlight late-breaking abstract at the ASCO Congress 2021, met its primary endpoint of disease-free survival with post nephrectomy adjuvant pembrolizumab vs. placebo in clear cell renal cell carcinoma patients. At ESMO 2021, the authors presented patient-reported outcomes, whereby no clinically meaningful changes from baseline in health-related quality of life or symptom scores were observed with adjuvant pembrolizumab or placebo. These findings suggest that adjuvant pembrolizumab was tolerable from a patient perspective. (3) A phase II prospective trial of frontline cabozantinib in metastatic collecting ducts carcinoma, namely the BONSAI trial (Meeturo 2), met its primary endpoint objective response rate, showing promising efficacy and acceptable tolerability of cabozantinib in respective patients. Since metastatic collecting ducts carcinoma is biologically poorly characterised and heavily underrepresented in prospective randomised trials, BONSAI gains particular importance.


Similar content being viewed by others
References
Cited literature
Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33.
Quhal F, Mori K, Bruchbacher A, et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Eur Urol Oncol. 2021;4(5):755–65.
Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15.
Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27.
ClinicalTrials.gov. Nivolumab combined with ipilimumab versus sunitinib in previously untreated advanced or metastatic renal cell carcinoma (checkmate 214). 2021. https://clinicaltrials.gov/ct2/show/NCT02231749. Accessed 26 Jan 2022.
Eloranta S, Smedby KE, Dickman PW, et al. Cancer survival statistics for patients and healthcare professionals—a tutorial of real-world data analysis. J Intern Med. 2021;289(1):12–28.
EU Clinical Trials Register. A randomised phase II trial of nivolumab in combination with alternatively scheduled ipilimumab in first-line treatment of patients with advanced or metastatic renal cell carcinoma (PRISM). 2018. https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-001476-33/GB. Accessed 26 Jan 2022.
Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(5):706–20.
ClinicalTrials.gov. Safety and efficacy study of pembrolizumab (MK-3475) as monotherapy in the adjuvant treatment of renal cell carcinoma post nephrectomy (MK-3475-564/KEYNOTE-564). 2021. https://clinicaltrials.gov/ct2/show/NCT03142334. Accessed 26 Jan 2022.
Smaldone MC, Fung C, Uzzo RG, et al. Adjuvant and neoadjuvant therapies in high-risk renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25(4):765–91.
Sun M, Marconi L, Eisen T, et al. Adjuvant vascular endothelial growth factor-targeted therapy in renal cell carcinoma: a systematic review and pooled analysis. Eur Urol. 2018;74(5):611–20.
Correa AF, Jegede O, Haas NB, et al. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation. J Clin Oncol. 2019;37(23):2062–71.
Ciszewski S, Jakimow A, Smolska-Ciszewska B. Collecting (Bellini) duct carcinoma: a clinical study of a rare tumour and review of the literature. Can Urol Assoc J. 2015;9(9–10):E589–E93.
Pagani F, Colecchia M, Sepe P, et al. Collecting ducts carcinoma: an orphan disease. Literature overview and future perspectives. Cancer Treat Rev. 2019;79:101891.
Further reading
ClinicalTrials.gov. caBozantinib in cOllectiNg ductS Renal Cell cArcInoma (BONSAI). 2021. https://clinicaltrials.gov/ct2/show/NCT03354884. Accessed 26 Jan 2022
Acknowledgements
None of the contributing authors have any conflicts of interest, including specific financial interests, relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
G.C. Hutterer and M. Pichler declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hutterer, G.C., Pichler, M. Renal cell carcinoma—presentation highlights from the ESMO Congress 2021. memo 15, 102–106 (2022). https://doi.org/10.1007/s12254-022-00798-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00798-6