Abstract
Background/purpose
Management of type 2 diabetes mellitus (T2DM) in patients with liver cirrhosis is complex and suboptimal, but no clinical trial has adequately investigated antidiabetic drug use for such patients. We evaluate the risk of mortality, cardiovascular events, and hepatic outcomes between dipeptidyl peptidase-4 (DPP-4) inhibitor users and nonusers in patients with type 2 diabetes mellitus (T2DM) and cirrhosis.
Methods
We selected 2828 paired propensity score matched DPP-4 inhibitor users and nonusers from a cohort of T2DM with compensated liver cirrhosis between January 1, 2007, and December 31, 2012. Cox proportional hazards models were used to assess the risk of main outcomes for DPP-4 inhibitor users.
Results
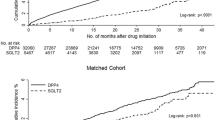
The incidence rate of decompensated cirrhosis during follow-up was 2.20 and 1.53 per 100 patient-years (adjusted hazard ratio [aHR] 1.35, 95% confidence interval [CI] 1.03–1.77) for DPP-4 inhibitor users and nonusers, respectively. The aHRs (95% CI) of variceal bleeding and hepatic failure were 1.67 (1.11–2.52) and 1.35 (1.02–1.79), respectively, for DPP-4 inhibitor users over nonusers. The risk of all-cause mortality, hepatocellular carcinoma, and major cardiovascular events between DPP-4 inhibitor users and nonusers were not statistically different.
Conclusions
This study found that DPP-4 inhibitor users were associated with higher risks of decompensated cirrhosis and hepatic failure than did nonusers among patients with T2DM and compensated liver cirrhosis. We must continue to search for appropriate antidiabetic drugs for patients with liver cirrhosis.


Similar content being viewed by others
References
Elkrief L, Rautou PE, Sarin S, Valla D, Paradis V, Moreau R. Diabetes mellitus in patients with cirrhosis: clinical implications and management. Liver Int. 2016;36:936–48.
Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019.
Brunton S. GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract. 2014;68:557–67.
Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, Terauchi Y, Nakajima A. Sitagliptin as a novel treatment agent for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103–5.
Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012;75:240–4.
Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215.
Ding KH, Zhong Q, Xu J, Isales CM. Glucose-dependent insulinotropic peptide: differential effects on hepatic artery vs portal vein endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E773–9.
Cheng M. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22:61–76.
Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104:157–63.
Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–4.
Mukerji AN, Patel V, Jain A. Improving survival in decompensated cirrhosis. Int J Hepatol. 2012;2012:318627.
Meduru P, Helmer D, Rajan M, Tseng CL, Pogach L, Sambamoorthi U. Chronic illness with complexity: implications for performance measurement of optimal glycemic control. J Gen Intern Med. 2007;22:408–18.
Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, Everson-Stewart S, Kinder L, Oliver M, Boyko EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and health care utilization. Am J Manag Care. 2008;14:15–23.
Wang X, Sarin SK, Ning Q. Definition of ACLF and inclusion criteria for extra-hepatic organ failure. Hepatol Int. 2015;9(3):360–5.
D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81.
Iezzoni LI. Risk adjustment for measuring healthcare outcomes. Chicago: Health Administration Press; 1997.
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1736–1788
Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. 2012;32:79–84.
Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res. 2018;122:1439–59.
Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90.
Gouverneur A, Lair A, Arnaud M, Begaud B, Raschi E, Pariente A, Salvo F. DPP-4 inhibitors and venous thromboembolism: an analysis of the WHO spontaneous reporting database. Lancet Diabetes Endocrinol. 2020;8:365–7.
Gupta AK, Verma AK, Kailashiya J, Singh SK, Kumar N. Sitagliptin: anti-platelet effect in diabetes and healthy volunteers. Platelets. 2012;23:565–70.
Ligueros-Saylan M, Foley JE, Schweizer A, Couturier A, Kothny W. An assessment of adverse effects of vildagliptin versus comparators on the liver, the pancreas, the immune system, the skin and in patients with impaired renal function from a large pooled database of Phase II and III clinical trials. Diabetes Obes Metab. 2010;12:495–509.
Asakawa M, Mitsui H, Akihisa M, Sekine T, Niitsu Y, Kobayashi A, Hashimoto N, Kawamura M, Ogawa Y. Efficacy and safety of sitagliptin for the treatment of diabetes mellitus complicated by chronic liver injury. Springerplus. 2015;4:346.
Langer DA, Shah VH. Nitric oxide and portal hypertension: interface of vasoreactivity and angiogenesis. J Hepatol. 2006;44:209–16.
Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27:57–64.
Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol. 2013;19:2298–306.
Yamamoto S, Tokuhara T, Nishikawa M, Nishizawa S, Nishioka T, Nozawa A, Takahashi A, Watanabe Y, Wada R, Wakasa K, et al. Spontaneous regression of hepatocellular carcinoma after improving diabetes mellitus: possibly responsible for immune system. Kanzo. 2012;53:164–7.
Gundling F, Seidl H, Strassen I, Haller B, Siegmund T, Umgelter A, Pehl C, Schepp W, Schumm-Draeger PM. Clinical manifestations and treatment options in patients with cirrhosis and diabetes mellitus. Digestion. 2013;87:75–84.
Harrison MF. The misunderstood coagulopathy of liver disease: a review for the acute setting. West J Emerg Med. 2018;19:863–71.
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This study was partially supported by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), Ministry of Science and Technology Clinical Trial Consortium for Stroke (MOST 108-2321-B-039-003-), and the Tseng-Lien Lin Foundation, Taichung, Taiwan. The writing or preparation of this paper was not funded by any organization; the data analyses were not undertaken by individuals who are employees of funders, or any author who received funding from funders; no writing support was from the funders. The corresponding authors had complete access to all data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: FSY, CMH, MCH and CCH; Methodology: JCW and CMH; Software: HTY; Validation: MCH and CCH; Formal analysis: HTY; Investigation: FSY and MCH; Resources: JCW; Data curation: CCH; Writing—original draft preparation: FSY; Writing—review and editing: MCH and CCH; Visualization: JCW; Supervision: CCH; Project administration: CMH; Funding acquisition: HTY. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical standards
This study was approved by the Research Ethics Committee of China Medical University and Hospital (CMUH104-REC2-115). All information identifying care providers or patients was encrypted, and we were permitted to waive informed consent.
Informed consent
To protect individual privacy, all patient or caregiver data were scrambled before being released. This study was approved by the Research Ethics Committee of China Medical University and Hospital (CMUH104-REC2-115-CR-4) and was exempted from informed consent requirements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yen, FS., Wei, J.CC., Yip, HT. et al. Dipeptidyl peptidase-4 inhibitors may accelerate cirrhosis decompensation in patients with diabetes and liver cirrhosis: a nationwide population-based cohort study in Taiwan. Hepatol Int 15, 179–190 (2021). https://doi.org/10.1007/s12072-020-10122-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10122-1