Abstract
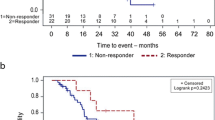
This prospective, single-arm study utilized alemtuzumab as a single agent in a novel maintenance schedule in previously treated chronic lymphocytic leukemia patients with the goal of delaying progression of disease and requirement for chemotherapy. In previously treated CLL patients who had achieved stable disease or better, the following schedule of subcutaneous alemtuzumab was administered: a dose escalation in the first week (3, 10 and 30 mg), followed by 7 weeks of 30 mg alemtuzumab once weekly, 16 weeks of 30 mg once every 2 weeks, followed by once every 3 weeks for 24 weeks. Thus, the entire duration of the planned treatment was 48 weeks. A total of 12 patients were enrolled 11 of which had at least one marker of poor prognosis (unmutated, Zap 70+, CD38+, del11q and del17p). The median chemotherapy-free interval was 13 months, and the median time to disease progression was 10 months. Three patients achieved a CR, one achieved nPR, one had a PR, five failed and two had shown a beneficial response but because of recurrent ITP had to stop alemtuzumab. In six of the 10 patients with previously relapsed disease, the chemotherapy-free interval was longer than their prior chemotherapy-free period. One patient had a reactivation of CMV antigenemia, and another had a bacterial pneumonia. There were no grade 3 or 4 toxicities. Alemtuzumab used in a maintenance schedule is a potentially safe and useful tool in delaying disease progression and chemotherapy-free intervals in previously treated CLL patients.
Similar content being viewed by others
References
Cheson BD, Bennett JM, Grever M, et al. National cancer institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–7.
Cancer Therapy Evaluation Program 1 Revised March 23, 1998. Common Toxicity Criteria, Version 2.0 DCTD, NCI, NIH, DHHS March 1998.
Keating MJ, O’Brien S, Kontoyiannis D, et al. Results of first salvage therapy for patients refractory to a fludarabine regimen in chronic lymphocytic leukemia. Leuk Lymph. 2002;43(9):1755–62.
Thieblemont C, Bouafia F, Hornez E, Dumontet C, Tartas S, Antal D, et al. Maintenance therapy with a monthly injection of alemtuzumab prolongs response duration in patients with refractory B-cell chronic lymphocytic leukemia/small lymphocytic lymphoma (B-CLL/SLL). Leuk Lymph. 2004;45:711–4.
Montillo M, Tedeschi A, et al. Alemtuzumab as consolidation after a response to fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukemia. J Clin Oncol. 2006;24:2337–42.
O’Brien SM, Kantarjian HM, Thomas DA, et al. Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer. 2003;98:2657–63.
Willis F, Marsh JC, Bevan DH, Killick SB, Lucas G, Griffiths R, et al. The effect of treatment with Campath-1H in patients with autoimmune cytopenias. Br J Haematol. 2001;114(4):891–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was made possible by an Investigator-sponsored research grant from Bayer Healthcare Pharmaceuticals, and partially supported by research grants from: The Karches Family Foundation,The Prince Family Foundation, The Marks Family Foundation, The Nash Family Foundation, The Leon Levy Foundation, and The Peter Jay Sharp Foundation.
Rights and permissions
About this article
Cite this article
Kaufman, M.S., Caramanica, A., Janson, D. et al. Alemtuzumab maintenance may safely prolong chemotherapy-free intervals in chronic lymphocytic leukemia. Med Oncol 28, 532–538 (2011). https://doi.org/10.1007/s12032-010-9478-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9478-3