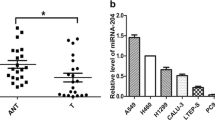
Abstract
MiR-132 is enriched in the central nerve system and is thought to be involved in neuronal development, maturation and function, and to be associated with several neurological disorders including Alzheimer’s disease. In addition to its documented neuronal functions, an emerging role for miR-132 in tumorigenesis has been suggested. Recently, hsa-miR-132 was shown to be modulated in different tumor types. However, its role in non-small cell lung cancer (NSCLC) remains unclear. Here, we show that hsa-miR-132 can initiate apoptosis in NSCLC cells to dramatically attenuate tumor formation in nude mice independent of its effect on the proliferation/apoptosis-associated gene, acetylcholinesterase (AChE). Interestingly, hsa-miR-132 has no pro-apoptotic effect in normal pulmonary trachea epithelium. Taken together, these results suggest that hsa-miR-132 represses NSCLC growth by inducing apoptosis independent of AChE.







Similar content being viewed by others
References
Babashah S, Soleimani M (2011) The oncogenic and tumour suppressive roles of microRNAs in cancer and apoptosis. European journal of cancer 47(8):1127–1137. doi:10.1016/j.ejca.2011.02.008
Battisti V, Schetinger MR, Maders LD, Santos KF, Bagatini MD, Correa MC, Spanevello RM, Do Carmo Araujo M, Morsch VM (2009) Changes in acetylcholinesterase (AchE) activity in lymphocytes and whole blood in acute lymphoblastic leukemia patients. Clinica chimica acta; international journal of clinical chemistry 402(1–2):114–118. doi:10.1016/j.cca.2008.12.030
Berson A, Knobloch M, Hanan M, Diamant S, Sharoni M, Schuppli D, Geyer BC, Ravid R, Mor TS, Nitsch RM, Soreq H (2008) Changes in readthrough acetylcholinesterase expression modulate amyloid-beta pathology. Brain : a journal of neurology 131(Pt 1):109–119. doi:10.1093/brain/awm276
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, Lao KQ, Livak KJ, Guegler KJ (2005) Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic acids research 33(20):e179. doi:10.1093/nar/gni178
Degterev A, Boyce M, Yuan J (2003) A decade of caspases. Oncogene 22(53):8543–8567. doi:10.1038/sj.onc.1207107
Favaloro B, Allocati N, Graziano V, Di Ilio C, De Laurenzi V (2012) Role of apoptosis in disease. Aging 4(5):330–349
Franca LB, Oliveira MA, Small IA, Zukin M, Araujo LH (2011) Adjuvant therapy for non-small cell lung cancer. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia 37(3):354–359
Friedlander RM (2003) Apoptosis and caspases in neurodegenerative diseases. The New England journal of medicine 348(14):1365–1375. doi:10.1056/NEJMra022366
Fuchs Y, Steller H (2011) Programmed cell death in animal development and disease. Cell 147(4):742–758. doi:10.1016/j.cell.2011.10.033
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J (2006) Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 24(28):4539–4544. doi:10.1200/JCO.2005.04.4859
Hacker G (2000) The morphology of apoptosis. Cell and tissue research 301(1):5–17
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP, Klag MJ, Whelton PK (2005) Major causes of death among men and women in China. The New England journal of medicine 353(11):1124–1134. doi:10.1056/NEJMsa050467
Jiang H, Zhang XJ (2008) Acetylcholinesterase and apoptosis. A novel perspective for an old enzyme. The FEBS journal 275(4):612–617. doi:10.1111/j.1742-4658.2007.06236.x
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nature reviews Molecular cell biology 2(8):589–598. doi:10.1038/35085008
Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH (2011) MicroRNA regulation of core apoptosis pathways in cancer. European journal of cancer 47(2):163–174. doi:10.1016/j.ejca.2010.11.005
Martinez-Moreno P, Nieto-Ceron S, Ruiz-Espejo F, Torres-Lanzas J, Tovar-Zapata I, Martinez-Hernandez P, Vidal CJ, Cabezas-Herrera J (2005) Acetylcholinesterase biogenesis is impaired in lung cancer tissues. Chemico-biological interactions 157–158:359–361
Ordonez NG (2003) The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. The American journal of surgical pathology 27(8):1031–1051
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell proliferation 45(6):487–498. doi:10.1111/j.1365-2184.2012.00845.x
Pabla N, Dong Z (2012) Curtailing side effects in chemotherapy: a tale of PKCdelta in cisplatin treatment. Oncotarget 3(1):107–111
Park SE, Kim ND, Yoo YH (2004) Acetylcholinesterase plays a pivotal role in apoptosome formation. Cancer research 64(8):2652–2655
Perry C, Sklan EH, Soreq H (2004) CREB regulates AChE-R-induced proliferation of human glioblastoma cells. Neoplasia 6(3):279–286. doi:10.1593/neo.3424
Qavi H, Al-Rajhi AA (2009) Acetylcholinesterase and HHV-8 in squamous cell carcinoma and retinoblastoma. In vivo 23(5):679–683
Sangha R, Price J, Butts CA (2010) Adjuvant therapy in non-small cell lung cancer: current and future directions. The oncologist 15(8):862–872. doi:10.1634/theoncologist.2009-0186
Saraste A, Pulkki K (2000) Morphologic and biochemical hallmarks of apoptosis. Cardiovascular research 45(3):528–537
Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H (2009) MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31(6):965–973. doi:10.1016/j.immuni.2009.09.019
Shore GC, Nguyen M (2008) Bcl-2 proteins and apoptosis: choose your partner. Cell 135(6):1004–1006. doi:10.1016/j.cell.2008.11.029
Soreq H (2008) Introduction: cholinesterases, from molecular complexity to non-hydrolytic functions. The FEBS journal 275(4):603. doi:10.1111/j.1742-4658.2007.06234.x
Soreq H, Seidman S (2001) Acetylcholinesterase—new roles for an old actor. Nature reviews Neuroscience 2(4):294–302. doi:10.1038/35067589
Syed M, Fenoglio-Preiser C, Skau KA, Weber GF (2008) Acetylcholinesterase supports anchorage independence in colon cancer. Clinical & experimental metastasis 25(7):787–798. doi:10.1007/s10585-008-9192-0
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267(5203):1456–1462
Thunnissen E, Kerr KM, Herth FJ, Lantuejoul S, Papotti M, Rintoul RC, Rossi G, Skov BG, Weynand B, Bubendorf L, Katrien G, Johansson L, Lopez-Rios F, Ninane V, Olszewski W, Popper H, Jaume S, Schnabel P, Thiberville L, Laenger F (2012) The challenge of NSCLC diagnosis and predictive analysis on small samples. Practical approach of a working group. Lung cancer 76(1):1–18. doi:10.1016/j.lungcan.2011.10.017
Toiber D, Berson A, Greenberg D, Melamed-Book N, Diamant S, Soreq H (2008) N-acetylcholinesterase-induced apoptosis in Alzheimer’s disease. PloS one 3(9):e3108. doi:10.1371/journal.pone.0003108
Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PA, Thum T, Groner B, Chowdhury K (2010) miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nature genetics 42(12):1101–1108. doi:10.1038/ng.709
Wanet A, Tacheny A, Arnould T, Renard P (2012) miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic acids research 40(11):4742–4753. doi:10.1093/nar/gks151
Wang Y, Lee CG (2009) MicroRNA and cancer–focus on apoptosis. Journal of cellular and molecular medicine 13(1):12–23. doi:10.1111/j.1582-4934.2008.00510.x
White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H (1994) Genetic control of programmed cell death in Drosophila. Science 264(5159):677–683
Xie J, Jiang H, Wan YH, Du AY, Guo KJ, Liu T, Ye WY, Niu X, Wu J, Dong XQ, Zhang XJ (2011) Induction of a 55 kDa acetylcholinesterase protein during apoptosis and its negative regulation by the Akt pathway. Journal of molecular cell biology 3(4):250–259. doi:10.1093/jmcb/mjq047
Xu D, Woodfield SE, Lee TV, Fan Y, Antonio C, Bergmann A (2009) Genetic control of programmed cell death (apoptosis) in Drosophila. Fly 3(1):78–90
Yang D, Li T, Wang Y, Tang Y, Cui H, Tang Y, Zhang X, Chen D, Shen N, Le W (2012) miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. Journal of cell science 125(Pt 7):1673–1682. doi:10.1242/jcs.086421
Ye W, Gong X, Xie J, Wu J, Zhang X, Ouyang Q, Zhao X, Shi Y, Zhang X (2010) AChE deficiency or inhibition decreases apoptosis and p53 expression and protects renal function after ischemia/reperfusion. Apoptosis : an international journal on programmed cell death 15(4):474–487. doi:10.1007/s10495-009-0438-3
Zhang XJ, Yang L, Zhao Q, Caen JP, He HY, Jin QH, Guo LH, Alemany M, Zhang LY, Shi YF (2002) Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell death and differentiation 9(8):790–800. doi:10.1038/sj.cdd.4401034
Zhao Y, Wang X, Wang T, Hu X, Hui X, Yan M, Gao Q, Chen T, Li J, Yao M, Wan D, Gu J, Fan J, He X (2011) Acetylcholinesterase, a key prognostic predictor for hepatocellular carcinoma, suppresses cell growth and induces chemosensitization. Hepatology 53(2):493–503. doi:10.1002/hep.24079
Acknowledgments
We thank Doctor Hongpin Zheng (Shanghai Institute of Biochemistry and Cell Biology, Shanghai, China) for proving dual luciferase reporter plasmid.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bo Zhang and Lu Lu are authors who contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Hsa-miR-132 triggers apoptosis in H520 cells. (a) Quantitative analysis of the percentage of sub-G1N520 cells following transfection with hsa-miR-132 mimics at the indicated time points post-transfection. (b) TUNEL assays of H520 at indicated time points post-transfection. Scale bar indicates 25 μm in length. (c) Quantitative analysis of TUNEL positive cells in (b). (d) Western blot of cleaved-PARP in H520 cells at indicated time points post-transfection. The results are presented as the mean ± SD. **p < 0.01 (JPEG 71 kb).
Supplementary Figure 2
Hsa-miR-132 triggers apoptosis in H460 cells. Western blot of (a) cleaved-PARP and (b) cleaved-caspase3/9 in H460 cells at the indicated time points following transfection. (JPEG 13 kb).
Rights and permissions
About this article
Cite this article
Zhang, B., Lu, L., Zhang, X. et al. Hsa-miR-132 Regulates Apoptosis in Non-Small Cell Lung Cancer Independent of Acetylcholinesterase. J Mol Neurosci 53, 335–344 (2014). https://doi.org/10.1007/s12031-013-0136-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-0136-z