Abstract
Purpose
Patients with familial hyperparathyroidism and low urinary calcium excretion may have familial hypocalciuric hypercalcemia (FHH) with mutations in one of three genes: the calcium-sensing receptor (CaSR) defining FHH-type 1, the adaptor-related protein complex 2 (AP2S1) related to FHH-type 3 or the G-protein subunit alpha11 (GNA11) associated with FHH-type 2. We aimed to evaluate the presence of mutations in these genes and to identify phenotypic specificities and differences in these patients.
Subjects and methods
Selected patients were recruited for genetic evaluation. After informed consent was signed, blood for DNA extraction was obtained and genetic sequencing of CaSR was done. In negative cases, we further performed sequencing of AP2S1 and GNA11.
Results
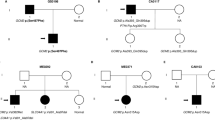
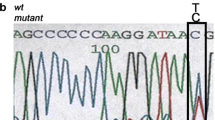
A total of 10 index cases were recruited. CaSR sequencing yielded three missense heterozygous mutations (30%): c.554G > A (p.I32V) previously characterized by our team, c.1394 G > A (p.R465Q) and a novel expected disease-causing mutation c.2479 A > C (p.S827R). We identified 2 additional patients (20%) carrying the deleterious recurrent mutation c.44G > T (p.R15L) in the AP2S1 gene. No GNA11 mutation was found. Clinically, patients with AP2S1 mutations had significant cognitive and behavioral disorders, and higher blood calcium and magnesium levels than patients with FHH1.
Conclusion
CaSR and AP2S1 sequencing is worthwhile in patients with familial hyperparathyroidism and phenotype suggesting FHH as it can diagnose up to 50% of cases. GNA11 mutations seem much rarer. Learning disabilities in these patients, associated with higher serum calcium and magnesium levels may suggest the presence of AP2S1 rather than CaSR mutation and may guide the first step in the genetic evaluation.




Similar content being viewed by others
References
R. Eastell, M.L. Brandi, A.G. Costa, P. D’Amour, D.M. Shoback, R.V. Thakker, Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab. 99(10), 3570–3579 (2014)
R.V. Thakker, Genetics of parathyroid tumours. J. Intern. Med. (2016) 280(6), 574–583
J. Varghese, T. Rich, C. Jimenez, Benign familial hypocalciuric hypercalcemia. Endocr. Pract. 17(Suppl 1), 13–17 (2011)
M.A. Nesbit, F.M. Hannan, S.A. Howles, V.N. Babinsky, R.A. Head, T. Cranston, N. Rust, M.R. Hobbs, H. Heath 3rd, R.V. Thakker, Mutations affecting G-protein subunit alpha11 in hypercalcemia and hypocalcemia. N. Eng. J. Med. 368(26), 2476–2486 (2013)
M.A. Nesbit, F.M. Hannan, S.A. Howles, A.A. Reed, T. Cranston, C.E. Thakker, L. Gregory, A.J. Rimmer, N. Rust, U. Graham, P.J. Morrison, S.J. Hunter, M.P. Whyte, G. McVean, D. Buck, R.V. Thakker, Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat. Genet. 45(1), 93–97 (2013)
A. Szalat, M. Shahar, S. Shpitzen, B. Nachmias, G. Munter, D. Gillis, R. Durst, D. Mevorach, E. Leitersdorf, V. Meiner, H. Rosen, Calcium-sensing receptor sequencing in 21 patients with idiopathic or familial parathyroid disorder: pitfalls and characterization of a novel I32 V loss-of-function mutation. Endocrine 48(2), 444–453 (2014)
I.A. Adzhubei, S. Schmidt, L. Peshkin, V.E. Ramensky, A. Gerasimova, P. Bork, A.S. Kondrashov, S.R. Sunyaev, A method and server for predicting damaging missense mutations. Nat. Methods 7(4), 248–249 (2010)
J.M. Schwarz, C. Rodelsperger, M. Schuelke, D. Seelow, Mutation Taster evaluates disease-causing potential of sequence alterations. Nat. Methods 7(8), 575–576 (2010)
S.E. Christensen, P.H. Nissen, P. Vestergaard, L. Heickendorff, K. Brixen, L. Mosekilde, Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods. Clin. Endocrinol. (Oxf). 69(5), 713–720 (2008)
C. Leech, P. Lohse, V. Stanojevic, A. Lechner, B. Goke, C. Spitzweg, Identification of a novel inactivating R465Q mutation of the calcium-sensing receptor. Biochem. Biophys. Res. Commun. 342(3), 996–1002 (2006)
N.F. Jakobsen, L. Rolighed, E. Moser, P.H. Nissen, L. Mosekilde, L. Rejnmark, Increased trabecular volumetric bone mass density in Familial Hypocalciuric Hypercalcemia (FHH) type 1: a cross-sectional study. Calcif. Tissue Int. 95(2), 141–152 (2014)
F.M. Hannan, M.A. Nesbit, C. Zhang, T. Cranston, A.J. Curley, B. Harding, C. Fratter, N. Rust, P.T. Christie, J.J. Turner, M.C. Lemos, M.R. Bowl, R. Bouillon, C. Brain, N. Bridges, C. Burren, J.M. Connell, H. Jung, E. Marks, D. McCredie, Z. Mughal, C. Rodda, S. Tollefsen, E.M. Brown, J.J. Yang, R.V. Thakker, Identification of 70 calcium-sensing receptor mutations in hyper- and hypo-calcaemic patients: evidence for clustering of extracellular domain mutations at calcium-binding sites. Hum. Mol. Genet. 21(12), 2768–2778 (2012)
Y. Fujisawa, R. Yamaguchi, E. Satake, K. Ohtaka, T. Nakanishi, K. Ozono, T. Ogata, Identification of AP2S1 mutation and effects of low calcium formula in an infant with hypercalcemia and hypercalciuria. J. Clin. Endocrinol. Metab. 98(12), E2022–E2027 (2013)
G.N. Hendy, L. Canaff, R.S. Newfield, L. Tripto-Shkolnik, B.Y. Wong, B.S. Lee, D.E. Cole, Codon Arg15 mutations of the AP2S1 gene: common occurrence in familial hypocalciuric hypercalcemia cases negative for calcium-sensing receptor (CASR) mutations. J. Clin. Endocrinol. Metab. 99(7), E1311–E1315 (2014)
S. Tenhola, G.N. Hendy, H. Valta, L. Canaff, B.S. Lee, B.Y. Wong, M.J. Valimaki, D.E. Cole, O. Makitie, Cinacalcet treatment in an adolescent with Concurrent 22q11.2 Deletion Syndrome and Familial Hypocalciuric Hypercalcemia Type 3 caused by AP2S1 mutation. J. Clin. Endocrinol. Metab. 100(7), 2515–2518 (2015)
S.A. Howles, F.M. Hannan, V.N. Babinsky, A. Rogers, C.M. Gorvin, N. Rust, T. Richardson, M.J. McKenna, M.A. Nesbit, R.V. Thakker, Cinacalcet for symptomatic hypercalcemia caused by AP2S1 mutations. N. Eng. J. Med. 374(14), 1396–1398 (2016)
F.M. Hannan, S.A. Howles, A. Rogers, T. Cranston, C.M. Gorvin, V.N. Babinsky, A.A. Reed, C.E. Thakker, D. Bockenhauer, R.S. Brown, J.M. Connell, J. Cook, K. Darzy, S. Ehtisham, U. Graham, T. Hulse, S.J. Hunter, L. Izatt, D. Kumar, M.J. McKenna, J.A. McKnight, P.J. Morrison, M.Z. Mughal, D. O’Halloran, S.H. Pearce, M.E. Porteous, M. Rahman, T. Richardson, R. Robinson, I. Scheers, H. Siddique, W.G. Van’t Hoff, T. Wang, M.P. Whyte, M.A. Nesbit, R.V. Thakker, Adaptor protein-2 sigma subunit mutations causing familial hypocalciuric hypercalcaemia type 3 (FHH3) demonstrate genotype-phenotype correlations, codon bias and dominant-negative effects. Hum. Mol. Genet. 24(18), 5079–5092 (2015)
R. Vargas-Poussou, L. Mansour-Hendili, S. Baron, J.P. Bertocchio, C. Travers, C. Simian, C. Treard, V. Baudouin, S. Beltran, F. Broux, O. Camard, S. Cloarec, C. Cormier, X. Debussche, E. Dubosclard, C. Eid, J.P. Haymann, S.R. Kiando, J.M. Kuhn, G. Lefort, A. Linglart, B. Lucas-Pouliquen, M.A. Macher, G. Maruani, S. Ouzounian, M. Polak, E. Requeda, D. Robier, C. Silve, J.C. Souberbielle, I. Tack, D. Vezzosi, X. Jeunemaitre, P. Houillier, Familial Hypocalciuric Hypercalcemia Types 1 and 3 and primary hyperparathyroidism: similarities and differences. J. Clin. Endocrinol. Metab. 101(5), 2185–2195 (2016)
H. Kahal, M. Aye, A.S. Rigby, T. Sathyapalan, R.J. England, S.L. Atkin, The effect of parathyroidectomy on neuropsychological symptoms and biochemical parameters in patients with asymptomatic primary hyperparathyroidism. Clin. Endocrinol. (Oxf). 76(2), 196–200 (2012)
D. Babinska, M. Barczynski, T. Stefaniak, T. Oseka, A. Babinska, D. Babinski, K. Sworczak, A.J. Lachinski, W. Nowak, Z. Sledzinski, Evaluation of selected cognitive functions before and after surgery for primary hyperparathyroidism. Langenbecks. Arch. Surg. 397(5), 825–831 (2012)
T. Weber, J. Eberle, U. Messelhauser, L. Schiffmann, C. Nies, J. Schabram, A. Zielke, K. Holzer, E. Rottler, D. Henne-Bruns, M. Keller, J. von Wietersheim, Parathyroidectomy, elevated depression scores, and suicidal ideation in patients with primary hyperparathyroidism: results of a prospective multicenter study. JAMA Surg. 148(2), 109–115 (2013)
H.T. McMahon, E. Boucrot, Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12(8), 517–533 (2011)
J.D. Shepherd, R.L. Huganir, The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 23, 613–643 (2007)
K.O. Schubert, M. Focking, J.H. Prehn, D.R. Cotter, Hypothesis review: are clathrin-mediated endocytosis and clathrin-dependent membrane and protein trafficking core pathophysiological processes in schizophrenia and bipolar disorder? Mol. Psychiatry 17(7), 669–681 (2012)
S. Matsuda, W. Kakegawa, T. Budisantoso, T. Nomura, K. Kohda, M. Yuzaki, Stargazin regulates AMPA receptor trafficking through adaptor protein complexes during long-term depression. Nat. Commun. 4, 2759 (2013)
W.F. Simonds, L.A. James-Newton, S.K. Agarwal, B. Yang, M.C. Skarulis, G.N. Hendy, S.J. Marx, Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 81(1), 1–26 (2002)
J. Warner, M. Epstein, A. Sweet, D. Singh, J. Burgess, S. Stranks, P. Hill, D. Perry-Keene, D. Learoyd, B. Robinson, P. Birdsey, E. Mackenzie, B.T. Teh, J.B. Prins, J. Cardinal, Genetic testing in familial isolated hyperparathyroidism: unexpected results and their implications. J. Med. Genet. 41(3), 155–160 (2004)
F.M. Hannan, M.A. Nesbit, P.T. Christie, C. Fratter, N.E. Dudley, G.P. Sadler, R.V. Thakker, Familial isolated primary hyperparathyroidism caused by mutations of the MEN1 gene. Nat. Clin. Pract. Endocrinol. Metab. 4(1), 53–58 (2008)
B. Guan, J.M. Welch, J.C. Sapp, H. Ling, Y. Li, J.J. Johnston, E. Kebebew, L.G. Biesecker, W.F. Simonds, S.J. Marx, S.K. Agarwal, GCM2-Activating mutations in familial isolated hyperparathyroidism. Am. J. Hum. Genet. 99(5), 1034–1044 (2016)
Acknowledgements
This study was supported by the Research Foundation of the Chief Scientist at the Israel Ministry of Health and by the Israel Endocrine Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Szalat, A., Shpitzen, S., Tsur, A. et al. Stepwise CaSR, AP2S1, and GNA11 sequencing in patients with suspected familial hypocalciuric hypercalcemia. Endocrine 55, 741–747 (2017). https://doi.org/10.1007/s12020-017-1241-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1241-5