Abstract
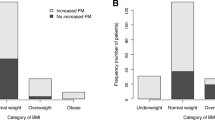
The prevalence of cardiac cachexia has previously been estimated to 8–42 %. However, novel treatment strategies for chronic heart failure (CHF) have improved and decreased morbidity and mortality. Therefore, we aimed to reassess the prevalence of cachexia in an outpatient CHF clinic and to characterize a CHF population with and without cachexia with respect to body composition and related biomarkers. From 2008 to 2011, we screened 238 optimally treated, non-diabetic CHF patients for cardiac cachexia, defined as unintentional non-oedematous weight loss of >5 % over ≥6 months. CHF patients (LVEF <45 %) with cachexia (n = 19) and without (n = 19) were compared to controls with prior myocardial infarction and left ventricular ejection fraction (LVEF) >45 % (n = 19). The groups were matched for age, sex, and kidney function. Body composition was assessed by dual energy X-ray absorptiometry. The prevalence of cachexia was 10.5 %. Abdominal fat ± SD (%) was reduced in cachectic CHF: 27.4 ± 10.0 versus 37.5 ± 10.6 % (CHF, no cachexia) and 40.6 ± 8.0 % (controls), (P < 0.001). NT-proBNP levels were inversely correlated to abdominal fat in a multivariate linear regression analysis adjusted for known predictors of NT-proBNP (LVEF and NYHA); (β = −0.28; P = 0.018). Myostatin levels were reduced in cachectic CHF compared to controls (P = 0.013). The prevalence of cachexia in stable CHF, treated according to recent guidelines, is lower than previously anticipated. Body alterations in cachexia consist mainly of reduced abdominal fat mass, and its inverse correlation to NT-proBNP suggests involvement of abdominal lipolysis. Our data do not support a role of circulating myostatin as a biomarker for muscle wasting.


Similar content being viewed by others
References
T.B. Horwich, G.C. Fonarow, M.A. Hamilton, W.R. MacLellan, M.A. Woo, J.H. Tillisch, The relationship between obesity and mortality in patients with heart failure. J. Am. Coll. Cardiol. 38, 789–795 (2001)
C.J. Lavie, A.F. Osman, R.V. Milani, M.R. Mehra, Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am. J. Cardiol. 91, 891–894 (2003)
A. Oreopoulos, R. Padwal, K. Kalantar-Zadeh, G.C. Fonarow, C.M. Norris, F.A. McAlister, Body mass index and mortality in heart failure: a meta-analysis. Am. Heart J. 156, 13–22 (2008)
S.J. Pocock, J.J. McMurray, J. Dobson, S. Yusuf, C.B. Granger, E.L. Michelson, J. Ostergren, M.A. Pfeffer, S.D. Solomon, S.D. Anker, K.B. Swedberg, Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur. Heart J. 29, 2641–2650 (2008)
S.D. Anker, P. Ponikowski, S. Varney, T.P. Chua, A.L. Clark, K.M. Webb-Peploe, D. Harrington, W.J. Kox, P.A. Poole-Wilson, A.J. Coats, Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349, 1050–1053 (1997)
H.S. Von, S.D. Anker, Cachexia as a major underestimated and unmet medical need: facts and numbers. J. Cachex. Sarcopenia. Muscle 1, 1–5 (2010)
S.D. Anker, A. Negassa, A.J. Coats, R. Afzal, P.A. Poole-Wilson, J.N. Cohn, S. Yusuf, Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 361, 1077–1083 (2003)
Effects of enalapril on mortality in severe congestive heart failure. Results of the cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N. Engl. J. Med. 316, 1429–1435 (1987)
P.A. Poole-Wilson, K. Swedberg, J.G. Cleland, L.A. Di, P. Hanrath, M. Komajda, J. Lubsen, B. Lutiger, M. Metra, W.J. Remme, C. Torp-Pedersen, A. Scherhag, A. Skene, Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 362, 7–13 (2003)
S.D. Anker, P.P. Ponikowski, A.L. Clark, F. Leyva, M. Rauchhaus, M. Kemp, M.M. Teixeira, P.G. Hellewell, J. Hooper, P.A. Poole-Wilson, A.J. Coats, Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur. Heart J. 20, 683–693 (1999)
F. Gustafsson, H. Ulriksen, H. Villadsen, H. Nielsen, B.B. Andersen, R. Hildebrandt, Prevalence and characteristics of heart failure clinics in Denmark—design of the Danish heart failure clinics network. Eur. J. Heart Fail. 7, 283–284 (2005)
C. Kistorp, J. Faber, S. Galatius, F. Gustafsson, J. Frystyk, A. Flyvbjerg, P. Hildebrandt, Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 112, 1756–1762 (2005)
M.A. Laskey, Dual-energy X-ray absorptiometry and body composition. Nutrition 12, 45–51 (1996)
M. Packer, A.J. Coats, M.B. Fowler, H.A. Katus, H. Krum, P. Mohacsi, J.L. Rouleau, M. Tendera, A. Castaigne, E.B. Roecker, M.K. Schultz, D.L. DeMets, Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 344, 1651–1658 (2001)
W.J. Evans, J.E. Morley, J. Argiles, C. Bales, V. Baracos, D. Guttridge, A. Jatoi, K. Kalantar-Zadeh, H. Lochs, G. Mantovani, D. Marks, W.E. Mitch, M. Muscaritoli, A. Najand, P. Ponikowski, F.F. Rossi, M. Schambelan, A. Schols, M. Schuster, D. Thomas, R. Wolfe, S.D. Anker, Cachexia: a new definition. Clin. Nutr. 27, 793–799 (2008)
M.A. Pfeffer, K. Swedberg, C.B. Granger, P. Held, J.J. McMurray, E.L. Michelson, B. Olofsson, J. Ostergren, S. Yusuf, S. Pocock, Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-overall programme. Lancet 362, 759–766 (2003)
M.J. Toth, S.S. Gottlieb, M.L. Fisher, E.T. Poehlman, Skeletal muscle atrophy and peak oxygen consumption in heart failure. Am. J. Cardiol. 79, 1267–1269 (1997)
H.S. Von, M. Lainscak, W. Doehner, P. Ponikowski, G. Rosano, J. Jordan, P. Rozentryt, M. Rauchhaus, R. Karpov, V. Tkachuk, Y. Parfyonova, A.Y. Zaritskey, E.V. Shlyakhto, J.G. Cleland, S.D. Anker, Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). J. Cachex. Sarcopenia. Muscle 1, 187–194 (2010)
M.R. Hoenig, Hypothesis: myostatin is a mediator of cardiac cachexia. Int. J. Cardiol. 124, 131–133 (2008)
N. Mangner, Y. Matsuo, G. Schuler, V. Adams, Cachexia in chronic heart failure: endocrine determinants and treatment perspectives. Endocrine (2012)
I. George, L.T. Bish, G. Kamalakkannan, C.M. Petrilli, M.C. Oz, Y. Naka, H.L. Sweeney, S. Maybaum, Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur. J. Heart Fail. 12, 444–453 (2010)
D. Gruson, S.A. Ahn, J.M. Ketelslegers, M.F. Rousseau, Increased plasma myostatin in heart failure. Eur. J. Heart Fail. 13, 734–736 (2011)
E. Zamora, R. Simo, J. Lupon, A. Galan, A. Urrutia, B. Gonzalez, D. Mas, V. Valle, Serum myostatin levels in chronic heart failure. Rev. Esp. Cardiol. 63, 992–996 (2010)
H.Q. Han, W.E. Mitch, Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr. Opin. Support. Palliat. Care 5, 334–341 (2011)
P. Dessi-Fulgheri, R. Sarzani, A. Rappelli, Role of the natriuretic peptide system in lipogenesis/lipolysis. Nutr. Metab Cardiovasc. Dis. 13, 244–249 (2003)
P.R. Kalra, S. Tigas, Regulation of lipolysis: natriuretic peptides and the development of cachexia. Int. J. Cardiol. 85, 125–132 (2002)
M. Lafontan, C. Moro, M. Berlan, F. Crampes, C. Sengenes, J. Galitzky, Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol. Metab. 19, 130–137 (2008)
J. Polak, M. Kotrc, Z. Wedellova, A. Jabor, I. Malek, J. Kautzner, L. Kazdova, V. Melenovsky, Lipolytic effects of B-type natriuretic peptide 1-32 in adipose tissue of heart failure patients compared with healthy controls. J. Am. Coll. Cardiol. 58, 1119–1125 (6-9-2011)
S.R. Das, M.H. Drazner, D.L. Dries, G.L. Vega, H.G. Stanek, S.M. Abdullah, R.M. Canham, A.K. Chung, D. Leonard, F.H. Wians Jr, J.A. de Lemos, Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 112, 2163–2168 (2005)
J.P. Araujo, P. Lourenco, F. Rocha-Goncalves, A. Ferreira, P. Bettencourt, Adiponectin is increased in cardiac cachexia irrespective of body mass index. Eur. J. Heart Fail. 11, 567–572 (2009)
M.B. McEntegart, B. Awede, M.C. Petrie, N. Sattar, F.G. Dunn, N.G. MacFarlane, J.J. McMurray, Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur. Heart J. 28, 829–835 (2007)
B. Schautz, W. Later, M. Heller, A. Peters, M.J. Muller, A. Bosy-Westphal. Impact of age on leptin and adiponectin independent of adiposity. Br. J. Nutr. 1–8 (2012)
Acknowledgments
Thanks to the nurses at the CHF outpatient clinic at the Herlev University Hospital for their invaluable help of recruiting patients and thanks to the laboratory technicians at the Metabolic Ward, O4. The work was financially supported by The Research Council of Herlev University Hospital and The A.P. Moeller Foundation for the Advancement of Medical Science.
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Christensen, H.M., Kistorp, C., Schou, M. et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine 43, 626–634 (2013). https://doi.org/10.1007/s12020-012-9836-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9836-3