Abstract
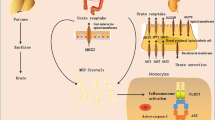
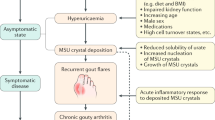
Acute gout is an auto-inflammatory disease characterized by self-limiting inflammation in response to the deposition of monosodium urate (MSU) crystals in the joints or tissues. Recognition of MSU triggers activation of the NLRP3 inflammasome, release of active interleukin (IL)-1β, and amplification of the inflammatory response by the surrounding tissue followed by recruitment and activation of inflammatory leukocytes. The shutdown of this inflammatory response is linked to a number of regulatory events ranging from crystal coating and apoptotic cell clearance through to pro-inflammatory cytokine regulation and transforming growth factor β1 (TGFβ1) production. This review will highlight mechanisms that limit acute inflammation triggered by MSU crystals and suggests areas for further research.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stamp LK, Wells JE, Pitama S, Faatoese A, et al. Hyperuricaemia and gout in New Zealand rural and urban Maori and non-Maori communities. Intern Med J. 2013;43(6):678–84.
Annemans L, Spaepen E, Gaskin M, Bonnemaire M, et al. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008;67(7):960–6.
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the national health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41.
Puig JG, Ruilope LM. Uric acid as a cardiovascular risk factor in arterial hypertension. J Hypertens. 1999;17(7):869–72.
Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, et al. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013;288(38):27138–49.
Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG. Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med. 1980;93(6):817–21.
Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res. 2010;62(2):170–80.
Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the third national health and nutrition examination survey. Arthritis Rheum. 2007;57(1):109–15.
Guerne PA, Terkeltaub R, Zuraw B, Lotz M. Inflammatory microcrystals stimulate interleukin-6 production and secretion by human monocytes and synoviocytes. Arthritis Rheum. 1989;32(11):1443–52.
di Giovine FS, Malawista SE, Thornton E, Duff GW. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. J Clin Invest. 1991;87(4):1375–81.
Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900.
Kozin F, Ginsberg MH, Skosey JL. Polymorphonuclear leukocyte responses to monosodium urate crystals: modification by adsorbed serum proteins. J Rheumatol. 1979;6(5):519–26.
Cherian PV, Schumacher Jr HR. Immunochemical and ultrastructural characterization of serum proteins associated with monosodium urate crystals (MSU) in synovial fluid cells from patients with gout. Ultrastruct Pathol. 1986;10(3):209–19.
Rosen MS, Baker DG, Schumacher Jr HR, Cherian PV. Products of polymorphonuclear cell injury inhibit IgG enhancement of monosodium urate-induced superoxide production. Arthritis Rheum. 1986;29(12):1473–9.
Terkeltaub R, Martin J, Curtiss LK, Ginsberg MH. Apolipoprotein B mediates the capacity of low density lipoprotein to suppress neutrophil stimulation by particulates. J Biol Chem. 1986;261(33):15662–7.
Terkeltaub RA, Dyer CA, Martin J, Curtiss LK. Apolipoprotein (apo) E inhibits the capacity of monosodium urate crystals to stimulate neutrophils. Characterization of intraarticular apo E and demonstration of apo E binding to urate crystals in vivo. J Clin Invest. 1991;87(1):20–6.
Landis RC, Yagnik DR, Florey O, Philippidis P, et al. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum. 2002;46(11):3026–33.
Martin WJ, Shaw O, Liu X, Steiger S, Harper JL. MSU crystal-recruited non-inflammatory monocytes differentiate into M1-like pro-inflammatory macrophages in a peritoneal murine model of gout. Arthritis Rheum. 2011;63(5):1322–32.
Urano W, Yamanaka H, Tsutani H, Nakajima H, et al. The inflammatory process in the mechanism of decreased serum uric acid concentrations during acute gouty arthritis. J Rheumatol. 2002;29(9):1950–3.
Scott P, Ma H, Viriyakosol, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006;177:6370–78.
Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83(3):865–75.
Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–8.
Steiger S, Harper JL. Neutrophil cannibalism triggers transforming growth factor beta1 production and self regulation of neutrophil inflammatory function in monosodium urate monohydrate crystal-induced inflammation in mice. Arthritis Rheum. 2013;65(3):815–23. Reports the phenomenon of neutrophil apoptosis and self clearance leading to TGFβ1 production and shutdown of MSU crystal-induced neutrophil pro-inflammatory functions in vivo.
Rose DM, Sydlaske AD, Agha-Babakhani A, Johnson K, Terkeltaub R. Transglutaminase 2 limits murine peritoneal acute gout-like inflammation by regulating macrophage clearance of apoptotic neutrophils. Arthritis Rheum. 2006;54(10):3363–71.
Fava R, Olsen N, Keski-Oja J, Moses H, Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989;169(1):291–6.
Scanu A, Oliviero F, Ramonda R, Frallonardo P, Dayer JM, Punzi L. Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor beta1 in the resolution phase. Ann Rheum Dis. 2012;71(4):621–4. Confirms the presence of increasing levels of TGFβ1 in the joints of patients during the resolution phase of a gout attack.
Chang SJ, Chen CJ, Tsai FC, Lai HM, et al. Associations between gout tophus and polymorphisms 869T/C and -509C/T in transforming growth factor beta1 gene. Rheumatology. 2008;47(5):617–21.
Liote F, Prudhommeaux F, Schiltz C, Champy R, et al. Inhibition and prevention of monosodium urate monohydrate crystal-induced acute inflammation in vivo by transforming growth factor beta1. Arthritis Rheum. 1996;39(7):1192–8.
Hsing AY, Kadomatsu K, Bonham MJ, Danielpour D. Regulation of apoptosis induced by transforming growth factor-beta1 in nontumorigenic rat prostatic epithelial cell lines. Cancer Res. 1996;56(22):5146–9.
Houde N, Chamoux E, Bisson M, Roux S. Transforming growth factor-beta1 (TGF-beta1) induces human osteoclast apoptosis by up-regulating Bim. J Biol Chem. 2009;284(35):23397–404.
Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol. 1995;154(5):2366–74.
Brinkmann V, Reichard U, Goosmann C, Fauler B, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5.
Schorn C, Janko C, Krenn V, Zhao Y, et al. Bonding the foe - NETting neutrophils immobilize the pro-inflammatory monosodium urate crystals. Front Immunol. 2012;3:376. Illustrates a link between NET formation and NETosis in the regulation of MSU crystal-induced inflammation in gout.
Schorn C, Janko C, Latzko M, Chaurio R, Schett G, Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol. 2012;3:277.
Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, et al. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6(12):e29318.
Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191(5):2647–56.
Fuchs TA, Abed U, Goosmann C, Hurwitz R, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41.
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41.
Dubois CM, Ruscetti FW, Palaszynski EW, Falk LA, Oppenheim JJ, Keller JR. Transforming growth factor beta is a potent inhibitor of interleukin 1 (IL-1) receptor expression: proposed mechanism of inhibition of IL-1 action. J Exp Med. 1990;172(3):737–44.
Redini F, Mauviel A, Pronost S, Loyau G, Pujol JP. Transforming growth factor beta exerts opposite effects from interleukin-1 beta on cultured rabbit articular chondrocytes through reduction of interleukin-1 receptor expression. Arthritis Rheum. 1993;36(1):44–50.
Chen CJ, Shi Y, Hearn A, Fitzgerald K, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116(8):2262–71.
Furst DE. Anakinra: review of recombinant human interleukin-I receptor antagonist in the treatment of rheumatoid arthritis. Clin Ther. 2004;26(12):1960–75.
Schiff MH. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann Rheum Dis. 2000;59:103–8.
Seckinger P, Klein-Nulend J, Alander C, Thompson RC, Dayer JM, Raisz LG. Natural and recombinant human IL-1 receptor antagonists block the effects of IL-1 on bone resorption and prostaglandin production. J Immunol. 1990;145(12):4181–4.
McColl SR, Paquin R, Menard C, Beaulieu AD. Human neutrophils produce high levels of the interleukin 1 receptor antagonist in response to granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J Exp Med. 1992;176(2):593–8.
Ulich TR, Yin SM, Guo KZ, del Castillo J, Eisenberg SP, Thompson RC. The intratracheal administration of endotoxin and cytokines. III. The interleukin-1 (IL-1) receptor antagonist inhibits endotoxin- and IL-1-induced acute inflammation. Am J Pathol. 1991;138(3):521–4.
Chen YH, Hsieh SC, Chen WY, Li KJ, et al. Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFbeta1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Ann Rheum Dis. 2011;70(9):1655–63. Demonstrates a role for intracellular cytokine regulators and sTNFR-I/II to suppress pro-inflammatory responses to MSU crystals during a gout attack.
Turner M, Chantry D, Katsikis P, Berger A, Brennan FM, Feldmann M. Induction of the interleukin 1 receptor antagonist protein by transforming growth factor-beta. Eur J Immunol. 1991;21(7):1635–9.
Wahl SM, Costa GL, Corcoran M, Wahl LM, Berger AE. Transforming growth factor-beta mediates IL-1-dependent induction of IL-1 receptor antagonist. J Immunol. 1993;150(8):3553–60.
Roberge CJ, de Medicis R, Dayer JM, Rola-Pleszczynski M, Naccache PH, Poubelle PE. Crystal-induced neutrophil activation. V. Differential production of biologically active IL-1 and IL-1 receptor antagonist. J Immunol. 1994;152(11):5485–94.
So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28.
Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68(10):1613–7.
Gratton SB, Scalapino KJ, Fye KH. Case of anakinra as a steroid-sparing agent for gout inflammation. Arthritis Rheum. 2009;61(9):1268–70.
Murakami Y, Akahoshi T, Kawai S, Inoue M, Kitasato H. Antiinflammatory effect of retrovirally transfected interleukin-10 on monosodium urate monohydrate crystal-induced acute inflammation in murine air pouches. Arthritis Rheum. 2002;46(9):2504–13.
Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–47.
Choe JY, Lee GH, Kim SK. Radiographic bone damage in chronic gout is negatively associated with the inflammatory cytokines soluble interleukin 6 receptor and osteoprotegerin. J Rheumatol. 2011;38(3):485–91.
Jones SA, Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta. 2002;1592(3):251–63.
Symons JA, Young PR, Duff GW. Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. PNAS. 1995;92(5):1714–8.
Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T. Negative regulation of cytokine signaling and immune responses by SOCS proteins. Arthritis Res Ther. 2005;7(3):100–10.
Bannenberg GL, Chiang N, Ariel A, Arita M, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174(7):4345–55.
Rajakariar R, Hilliard M, Lawrence T, Trivedi S, Colville-Nash P, Bellingan G, et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci U S A. 2007;104(52):20979–84.
Scher JU, Pillinger MH. The anti-inflammatory effects of prostaglandins. J Investig Med. 2009;57(6):703–8.
Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–27.
Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73.
Murakami Y, Akahoshi T, Hayashi I, Endo H, Hashimoto A, Kono S, et al. Inhibition of monosodium urate monohydrate crystal-induced acute inflammation by retrovirally transfected prostaglandin D synthase. Arthritis Rheum. 2003;48(10):2931–41.
Akahoshi T, Namai R, Murakami Y, Watanabe M, Matsui T, Nishimura A, et al. Rapid induction of peroxisome proliferator-activated receptor gamma expression in human monocytes by monosodium urate monohydrate crystals. Arthritis Rheum. 2003;48(1):231–9.
Jung SM, Schumacher HR, Kim H, Kim M, Lee SH, Pessler F. Reduction of urate crystal-induced inflammation by root extracts from traditional oriental medicinal plants: elevation of prostaglandin D2 levels. Arthritis Res Ther. 2007;9(4):R64.
Yan Y, Jiang W, Spinetti T, Tardivel A, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38(6):1154–63. Reports omega-3 fatty acids able to suppress NLRP3 inflammasome-mediated inflammation. Provides a rationale for a possible role for pro-resolution lipid mediators in the resolution of NLRP3-driven gout attacks.
Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–7.
Compliance with Ethics Guidelines
Conflict of Interest
Stefanie Steiger and Jacquie L. Harper declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Crystal Arthritis
Rights and permissions
About this article
Cite this article
Steiger, S., Harper, J.L. Mechanisms of Spontaneous Resolution of Acute Gouty Inflammation. Curr Rheumatol Rep 16, 392 (2014). https://doi.org/10.1007/s11926-013-0392-5
Published:
DOI: https://doi.org/10.1007/s11926-013-0392-5