Abstract
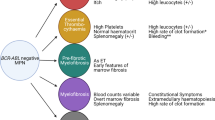
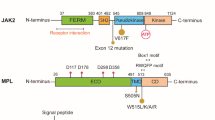
During the past 10 years, major progress has been accomplished with the discovery of activating mutations that are associated with the vast majority of BCR-ABL negative human myeloproliferative neoplasms (MPNs). The identification in 2005 of JAK2 V617F triggered great interest in the JAK2-STAT5/STAT3 pathway. Discovery in 2006 of mutants of thrombopoietin receptor (TPO-R/MPL) and later on of mutants in negative regulators of JAK-STAT pathway led to the notion that persistent JAK2 activation is a hallmark of MPNs. In 2013, mutations in the gene coding for the chaperone calreticulin were reported in 20–30 % of essential thrombocythemia and primary myelofibrosis patients. Here, we will address the question: what do we know about calreticulin that could help us understand its role in MPNs? In addition to oncogenic driver mutations, certain MPNs also exhibit epigenetic mutations. Targeting of both oncogenic drivers and epigenetic defects could be required for effective therapy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32(21):2601–13. doi:10.1038/onc.2012.347.
Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O, Hubbard SR. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012;19(8):754–9. doi:10.1038/nsmb.2348. This study describes JAK2 V617F pseudokinase domain.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–90. doi:10.1056/NEJMoa1311347. This study describes CALR mutations in MPN for the first time.
Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–405. doi:10.1056/NEJMoa1312542. This study describes CALR mutations in MPN for the first time.
Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123(22):e123–33. doi:10.1182/blood-2014-02-554634. This study provides a comprehensive genomic and transcriptomic analysis of MPN.
Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356(5):459–68. doi:10.1056/NEJMoa065202.
James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. doi:10.1038/nature03546.
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. doi:10.1016/j.ccr.2005.03.023.
Kralovics R, Teo SS, Buser AS, Brutsche M, Tiedt R, Tichelli A, et al. Altered gene expression in myeloproliferative disorders correlates with activation of signaling by the V617F mutation of Jak2. Blood. 2005;106(10):3374–6. doi:10.1182/blood-2005-05-1889.
Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. doi:10.1016/S0140-6736(05)71142-9.
Dusa A, Staerk J, Elliott J, Pecquet C, Poirel HA, Johnston JA, et al. Substitution of pseudokinase domain residue Val-617 by large non-polar amino acids causes activation of JAK2. J Biol Chem. 2008;283(19):12941–8. doi:10.1074/jbc.M709302200.
Dusa A, Mouton C, Pecquet C, Herman M, Constantinescu SN. JAK2 V617F constitutive activation requires JH2 residue F595: a pseudokinase domain target for specific inhibitors. PLoS One. 2010;5(6):e11157. doi:10.1371/journal.pone.0011157.
Silvennoinen O, Hubbard SR. Molecular insights into regulation of JAK2 in myeloproliferative neoplasms. Blood. 2015. doi:10.1182/blood-2015-01-621110.
Sangkhae V, Etheridge SL, Kaushansky K, Hitchcock IS. The thrombopoietin receptor, MPL, is critical for development of a JAK2V617F-induced myeloproliferative neoplasm. Blood. 2014;124(26):3956–63. doi:10.1182/blood-2014-07-587238.
Walz C, Ahmed W, Lazarides K, Betancur M, Patel N, Hennighausen L, et al. Essential role for Stat5a/b in myeloproliferative neoplasms induced by BCR-ABL1 and JAK2(V617F) in mice. Blood. 2012;119(15):3550–60. doi:10.1182/blood-2011-12-397554.
Yan D, Hutchison RE, Mohi G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood. 2012;119(15):3539–49. doi:10.1182/blood-2011-03-345215.
Moucadel V, Constantinescu SN. Differential STAT5 signaling by ligand-dependent and constitutively active cytokine receptors. J Biol Chem. 2005;280(14):13364–73. doi:10.1074/jbc.M407326200.
Girardot M, Pecquet C, Boukour S, Knoops L, Ferrant A, Vainchenker W, et al. miR-28 is a thrombopoietin receptor targeting microRNA detected in a fraction of myeloproliferative neoplasm patient platelets. Blood. 2010;116(3):437–45. doi:10.1182/blood-2008-06-165985.
Girardot M, Pecquet C, Chachoua I, Van Hees J, Guibert S, Ferrant A, et al. Persistent STAT5 activation in myeloid neoplasms recruits p53 into gene regulation. Oncogene. 2014. doi:10.1038/onc.2014.60.
Duek A, Lundberg P, Shimizu T, Grisouard J, Karow A, Kubovcakova L, et al. Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F-driven mouse model of MPNs. Blood. 2014;123(25):3943–50. doi:10.1182/blood-2013-07-514208.
Grisouard J, Shimizu T, Duek A, Kubovcakova L, Hao-Shen H, Dirnhofer S, et al. Deletion of Stat3 in hematopoietic cells enhances thrombocytosis and shortens survival in a JAK2-V617F mouse model of MPN. Blood. 2015. doi:10.1182/blood-2014-08-594572.
Chen E, Ahn JS, Massie CE, Clynes D, Godfrey AL, Li J, et al. JAK2V617F promotes replication fork stalling with disease-restricted impairment of the intra-S checkpoint response. Proc Natl Acad Sci U S A. 2014;111(42):15190–5. doi:10.1073/pnas.1401873111.
Lundberg P, Takizawa H, Kubovcakova L, Guo G, Hao-Shen H, Dirnhofer S, et al. Myeloproliferative neoplasms can be initiated from a single hematopoietic stem cell expressing JAK2-V617F. J Exp Med. 2014;211(11):2213–30. doi:10.1084/jem.20131371.
Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010;17(6):584–96. doi:10.1016/j.ccr.2010.05.015.
Kubovcakova L, Lundberg P, Grisouard J, Hao-Shen H, Romanet V, Andraos R, et al. Differential effects of hydroxyurea and INC424 on mutant allele burden and myeloproliferative phenotype in a JAK2-V617F polycythemia vera mouse model. Blood. 2013;121(7):1188–99. doi:10.1182/blood-2012-03-415646.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. doi:10.1371/journal.pmed.0030270.
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M, et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472–6. doi:10.1182/blood-2006-04-018879.
Staerk J, Lacout C, Sato T, Smith SO, Vainchenker W, Constantinescu SN. An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor. Blood. 2006;107(5):1864–71. doi:10.1182/blood-2005-06-2600.
Defour JP, Itaya M, Gryshkova V, Brett IC, Pecquet C, Sato T, et al. Tryptophan at the transmembrane-cytosolic junction modulates thrombopoietin receptor dimerization and activation. Proc Natl Acad Sci U S A. 2013;110(7):2540–5. doi:10.1073/pnas.1211560110.
Pecquet C, Staerk J, Chaligne R, Goss V, Lee KA, Zhang X, et al. Induction of myeloproliferative disorder and myelofibrosis by thrombopoietin receptor W515 mutants is mediated by cytosolic tyrosine 112 of the receptor. Blood. 2010;115(5):1037–48. doi:10.1182/blood-2008-10-183558.
Dudek-Peric AM, Ferreira GB, Muchowicz A, Wouters J, Prada N, Martin S, et al. Antitumor immunity triggered by melphalan is potentiated by melanoma cell surface-associated calreticulin. Cancer Res. 2015;75(8):1603–14. doi:10.1158/0008-5472.CAN-14-2089.
Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol. 2012;44(6):842–6. doi:10.1016/j.biocel.2012.02.009.
Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–34. doi:10.1016/j.cell.2005.08.032.
Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013;24(4):311–8. doi:10.1016/j.cytogfr.2013.05.001.
Tannous A, Pisoni GB, Hebert DN, Molinari M. N-linked sugar-regulated protein folding and quality control in the ER. Semin Cell Dev Biol. 2014. doi:10.1016/j.semcdb.2014.12.001.
Hebert DN, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81(3):425–33.
Wijeyesakere SJ, Rizvi SM, Raghavan M. Glycan-dependent and -independent interactions contribute to cellular substrate recruitment by calreticulin. J Biol Chem. 2013;288(49):35104–16. doi:10.1074/jbc.M113.507921.
Kozlov G, Pocanschi CL, Rosenauer A, Bastos-Aristizabal S, Gorelik A, Williams DB, et al. Structural basis of carbohydrate recognition by calreticulin. J Biol Chem. 2010;285(49):38612–20. doi:10.1074/jbc.M110.168294.
Chouquet A, Paidassi H, Ling WL, Frachet P, Houen G, Arlaud GJ, et al. X-ray structure of the human calreticulin globular domain reveals a peptide-binding area and suggests a multi-molecular mechanism. PLoS One. 2011;6(3):e17886. doi:10.1371/journal.pone.0017886.
Marty C, Harini N, Pecquet C, Chachoua I, Gryshkova V, Villeval JL, et al. (2014) Calr Mutants Retroviral Mouse Models Lead to a Myeloproliferative Neoplasm Mimicking an Essential Thrombocythemia Progressing to a Myelofibrosis Blood (ASH Annual Meeting Abstracts) 124 (21):Abstract 157
Rumi E, Pietra D, Ferretti V, Klampfl T, Harutyunyan AS, Milosevic JD, et al. JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–51. doi:10.1182/blood-2013-11-539098.
Broseus J, Park JH, Carillo S, Hermouet S, Girodon F. Presence of calreticulin mutations in JAK2-negative polycythemia vera. Blood. 2014;124(26):3964–6. doi:10.1182/blood-2014-06-583161.
Cabagnols X, Cayuela JM, Vainchenker W. A CALR mutation preceding BCR-ABL1 in an atypical myeloproliferative neoplasm. N Engl J Med. 2015;372(7):688–90. doi:10.1056/NEJMc1413718.
Passamonti F, Caramazza D, Maffioli M. JAK inhibitor in CALR-mutant myelofibrosis. N Engl J Med. 2014;370(12):1168–9. doi:10.1056/NEJMc1400499#SA1.
Cassinat B, Verger E, Kiladjian JJ. Interferon alfa therapy in CALR-mutated essential thrombocythemia. N Engl J Med. 2014;371(2):188–9. doi:10.1056/NEJMc1401255.
Kollmann K, Nangalia J, Warsch W, Quentmeier H, Bench A, Boyd E, et al. MARIMO cells harbor a CALR mutation but are not dependent on JAK2/STAT5 signaling. Leukemia. 2014. doi:10.1038/leu.2014.285.
Yoshida H, Kondo M, Ichihashi T, Hashimoto N, Inazawa J, Ohno R, et al. A novel myeloid cell line, Marimo, derived from therapy-related acute myeloid leukemia during treatment of essential thrombocythemia: consistent chromosomal abnormalities and temporary C-MYC gene amplification. Cancer Genet Cytogenet. 1998;100(1):21–4.
Cabagnols X, Defour JP, Ugo V, Ianotto JC, Mossuz P, Mondet J, et al. (2014) Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia Sep 19. doi: 10.1038/leu.2014.270
Rumi E, Pietra D, Pascutto C, Guglielmelli P, Martinez-Trillos A, Casetti I, et al. Clinical effect of driver mutations of JAK2, CALR, or MPL in primary myelofibrosis. Blood. 2014;124(7):1062–9. doi:10.1182/blood-2014-05-578435.
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–13. doi:10.1182/blood-2014-05-579136.
Tefferi A, Lasho TL, Tischer A, Wassie EA, Finke CM, Belachew AA, et al. The prognostic advantage of calreticulin mutations in myelofibrosis might be confined to type 1 or type 1-like CALR variants. Blood. 2014;124(15):2465–6. doi:10.1182/blood-2014-07-588426.
Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–8. doi:10.1182/blood-2013-11-537167. This study provides a comprehensive analysis of somatic mutations in MPN.
Rampal R, Ahn J, Abdel-Wahab O, Nahas M, Wang K, Lipson D, et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A. 2014;111(50):E5401–10. doi:10.1073/pnas.1407792111.
Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–301. doi:10.1056/NEJMoa0810069.
Delatte B, Deplus R, Fuks F. Playing TETris with DNA modifications. EMBO J. 2014;33(11):1198–211. doi:10.15252/embj.201488290.
Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, et al. Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601–12. doi:10.1056/NEJMoa1412098.
Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11–24. doi:10.1016/j.ccr.2011.06.001.
Kameda T, Shide K, Yamaji T, Kamiunten A, Sekine M, Taniguchi Y, et al. Loss of TET2 has dual roles in murine myeloproliferative neoplasms: disease sustainer and disease accelerator. Blood. 2015;125(2):304–15. doi:10.1182/blood-2014-04-555508.
Tefferi A, Pardanani A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia. 2009;23(5):905–11. doi:10.1038/leu.2009.47.
Abdel-Wahab O, Pardanani A, Rampal R, Lasho TL, Levine RL, Tefferi A. DNMT3A mutational analysis in primary myelofibrosis, chronic myelomonocytic leukemia and advanced phases of myeloproliferative neoplasms. Leukemia. 2011;25(7):1219–20. doi:10.1038/leu.2011.82.
Ernst T, Chase AJ, Score J, Hidalgo-Curtis CE, Bryant C, Jones AV, et al. Inactivating mutations of the histone methyltransferase gene EZH2 in myeloid disorders. Nat Genet. 2010;42(8):722–6. doi:10.1038/ng.621.
Carbuccia N, Murati A, Trouplin V, Brecqueville M, Adelaide J, Rey J, et al. Mutations of ASXL1 gene in myeloproliferative neoplasms. Leukemia. 2009;23(11):2183–6. doi:10.1038/leu.2009.141.
Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med. 2010;362(4):369–70. doi:10.1056/NEJMc0910063.
Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488(7413):656–9. doi:10.1038/nature11323.
Harutyunyan A, Klampfl T, Cazzola M, Kralovics R. p53 lesions in leukemic transformation. N Engl J Med. 2011;364(5):488–90. doi:10.1056/NEJMc1012718.
Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18(11):3008–14. doi:10.1158/1078-0432.CCR-11-3145.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98. doi:10.1056/NEJMoa1110556.This study is one of the two COMFORT trials reporting the efficacy of ruxolitinib.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi:10.1056/NEJMoa1110557. This study is one of the two COMFORT trials reporting the efficacy of ruxolitinib.
Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–35. doi:10.1056/NEJMoa1409002. This study is the RESPONSE trial.
Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489(7414):155–9. doi:10.1038/nature11303.
Manshouri T, Estrov Z, Quintas-Cardama A, Burger J, Zhang Y, Livun A, et al. Bone marrow stroma-secreted cytokines protect JAK2(V617F)-mutated cells from the effects of a JAK2 inhibitor. Cancer Res. 2011;71(11):3831–40. doi:10.1158/0008-5472.CAN-10-4002.
Choong ML, Pecquet C, Pendharkar V, Diaconu CC, Yong JW, Tai SJ, et al. Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors. J Cell Mol Med. 2013;17(11):1397–409. doi:10.1111/jcmm.12156.
Bartalucci N, Tozzi L, Bogani C, Martinelli S, Rotunno G, Villeval JL, et al. Co-targeting the PI3K/mTOR and JAK2 signalling pathways produces synergistic activity against myeloproliferative neoplasms. J Cell Mol Med. 2013;17(11):1385–96. doi:10.1111/jcmm.12162.
Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R, et al. Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood. 2014;123(13):2075–83. doi:10.1182/blood-2014-01-547760.
Scott LM. The JAK2 exon 12 mutations: a comprehensive review. Am J Hematol. 2011;86(8):668–76. doi:10.1002/ajh.22063.
Acknowledgments
Support from the Ludwig Institute for Cancer Research, FRS-FNRS Belgium, Programs IAP BCHM61B5, the Action de Recherche Concertée projects ARC10/15-027 and MEXP31C1, Salus Sanguinis Foundation and MPN Foundation USA is acknowledged. XC is a post-doctoral fellow of the de Duve Institute.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Xavier Cahu declares no potential conflicts of interest. Stefan N. Constantinescu is a board member for Dafra Pharma, Belgium, Personal Genetics, Romania, Novartis, Myelofibrosis Board, and Shire, Ad-Hoc Anagrelide; is a consultant for Novartis, Teva, and Shire; reports a grant from Aventis; and payment for the development of educational presentations including service on speakers’ bureaus and travel/accommodations expenses covered or reimbursed from Teva, Shire, and Novartis.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Myeloproliferative Disorders
Rights and permissions
About this article
Cite this article
Cahu, X., Constantinescu, S.N. Oncogenic Drivers in Myeloproliferative Neoplasms: From JAK2 to Calreticulin Mutations. Curr Hematol Malig Rep 10, 335–343 (2015). https://doi.org/10.1007/s11899-015-0278-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-015-0278-x