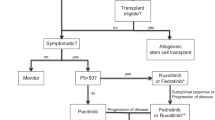
Opinion statement
Seven years after the approval of the Janus kinase 1/2 (JAK1/2) inhibitor ruxolitinib, it remains the only drug licensed for the treatment of myelofibrosis. Patients who discontinue ruxolitinib have a dismal outcome, and this is, therefore, an area of significant unmet need. Given the central role that JAK-signal transducer and activator of transcription (STAT) activation plays in disease pathogenesis, there have been many other JAK inhibitors tested, but most have been abandoned, for a variety of reasons. The JAK2-selective inhibitor fedratinib has recently been resurrected, and there has been a resurgence of interest in the failed JAK1/2 inhibitor momelotinib, which possibly improves anemia. Pacritinib, a non-myelosuppressive JAK2-selective inhibitor, is currently in a dose-ranging study mandated by regulatory authorities. A plethora of other targeted agents, most backed by preclinical data, are in various stages of investigation. These include epigenetic and immune therapies, agents targeting cellular survival, metabolic and apoptotic pathways, the cell cycle, DNA repair, and protein folding and degradation, among others. However, at this time, none of these is close to registration or even in a pivotal trial, illustrating the difficulties in recapitulating the clinical disease in preclinical models. Most current clinical trials are testing the addition of a novel agent to ruxolitinib, either in the frontline setting or in the context of an insufficient response to ruxolitinib, or attempting to study new drugs in the second-line, “ruxolitinib failure” setting. Emerging data supports the addition of azacitidine to ruxolitinib in some patients. Other strategies have focused on improving cytopenias, through amelioration of bone marrow fibrosis or other mechanisms. This is important, because cytopenias are the commonest reason for ruxolitinib interruption and/or dose reduction, and dose optimization of ruxolitinib is tied to its survival benefit. The activin receptor ligand trap, sotatercept, and the anti-fibrotic agent, PRM-151, have shown promise in this regard.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Verstovsek S, Mesa RA, Gotlib J, Gupta V, Di Persio JF, Catalano JV, et al. COMFORT-I investigators. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10:55–017-0417-z The 5-year update of the COMFORT-1 trial that led to the approval of ruxolitinib for myelofibrosis.
•• Harrison CN, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701–7 The 5-year update of the COMFORT-2 trial that led to the approval of ruxolitinib for myelofibrosis.
Kuykendall AT, Shah S, Talati C, Al Ali N, Sweet K, Padron E, et al. Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann Hematol. 2018;97:435–41.
• Newberry KJ, Patel K, Masarova L, Luthra R, Manshouri T, Jabbour E, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood. 2017;130:1125–31 The first paper to report on the outcomes of patients with advanced myelofibrosis who discontinue ruxolitinib.
Barosi G, Zhang MJ, Peter GR. Does ruxolitinib improve survival of persons with MPN-associated myelofibrosis? Should it? Leukemia. 2014;28:2267–70.
Cervantes F, Pereira A. Does ruxolitinib prolong the survival of patients with myelofibrosis? Blood. 2017;129:832–7.
Miller CB, Komrokji RS, Mesa RA, Sun W, Montgomery M, Verstovsek S. Practical measures of clinical benefit with ruxolitinib therapy: an exploratory analysis of COMFORT-I. Clin Lymphoma Myeloma Leuk. 2017;17:479–87.
Verstovsek S, Kantarjian HM, Estrov Z, Cortes JE, Thomas DA, Kadia T, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120:1202–9.
Vannucchi AM, Kantarjian HM, Kiladjian JJ, Gotlib J, Cervantes F, Mesa RA, et al. A pooled analysis of overall survival in COMFORT-I and COMFORT-II, 2 randomized phase 3 trials of ruxolitinib for the treatment of myelofibrosis. Haematologica. 2015;100:1139–45.
Bose P, Verstovsek S. Management of myelofibrosis-related cytopenias. Curr Hematol Malig Rep. 2018 Jun;13(3):164–72.
Passamonti F, Cervantes F, Vannucchi AM, Morra E, Rumi E, Pereira A, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115:1703–8.
Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–7.
Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807.
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–98.
Gupta V, Harrison C, Hexner EO, Al-Ali HK, Foltz L, Montgomery M, et al. The impact of anemia on overall survival in patients with myelofibrosis treated with ruxolitinib in the COMFORT studies. Haematologica. 2016;101:e482–4.
Al-Ali HK, Stalbovskaya V, Gopalakrishna P, Perez-Ronco J, Foltz L. Impact of ruxolitinib treatment on the hemoglobin dynamics and the negative prognosis of anemia in patients with myelofibrosis. Leuk Lymphoma. 2016;57:2464–7.
Malak S, Cony-Makhoul P, Ianotto J, Ranta D, Rodon P, Vacheret F, et al. Efficacy and safety of erythropoietic-stimulating agents with ruxolitinib in myelofibrosis patients: a retrospective analysis on 45 patients. On Behalf of the French Intergroup of Myeloproliferative Disorders (FIM). Blood. 2016;128:3123.
McMullin MF, Harrison CN, Niederwieser D, Demuynck H, Jakel N, Gopalakrishna P, et al. The use of erythropoiesis-stimulating agents with ruxolitinib in patients with myelofibrosis in COMFORT-II: an open-label, phase 3 study assessing efficacy and safety of ruxolitinib versus best available therapy in the treatment of myelofibrosis. Exp Hematol Oncol. 2015;4:26–015–0021-2 eCollection 2015.
Crisa E, Cilloni D, Elli EM, Martinelli V, Palumbo GA, Pugliese N, et al. The use of erythropoiesis-stimulating agents is safe and effective in the management of anaemia in myelofibrosis patients treated with ruxolitinib. Br J Haematol. 2018;182:701–4.
Daver N, Cortes J, Newberry K, Jabbour E, Zhou L, Wang X, et al. Ruxolitinib in combination with lenalidomide as therapy for patients with myelofibrosis. Haematologica. 2015;100:1058–63.
Stegelmann F, Hebart H, Bangerter M, Wolleschak D, Griesshammer M, Koschmieder S, et al. Ruxolitinib plus pomalidomide in myelofibrosis: updated results from the Mpnsg-0212 Trial (NCT01644110). Blood. 2016;128:1939.
Harrison CN, Kiladjian JJ, Heidel FH, Vannucchi AM, Passamonti F, Hayat A, et al. Efficacy, safety, and confirmation of the recommended phase 2 starting dose of the combination of ruxolitinib (RUX) and panobinostat (PAN) in patients (Pts) with myelofibrosis (MF). Blood. 2015;126:4060.
Bose P, Pemmaraju N, Schroeder K, Ferrajoli A, Jabbour EJ, Daver N, et al. Phase 2 study of pracinostat in combination with ruxolitinib in patients (pts) with myelofibrosis (MF). Blood. 2017;130:1632.
Durrant S, Nagler A, Vannucchi AM, Lavie D, Chuah C, Passamonti F, et al. An open-label, multicenter, 2-arm, dose-finding, phase 1b study of the combination of ruxolitinib and buparlisib (BKM120) in patients with myelofibrosis: results from HARMONY Study. Blood. 2015;126:827.
• Masarova L, Verstovsek S, Cortes JE, Pemmaraju N, Bose P, Ohanian MN, et al. Updated results of phase 2 study of ruxolitinib in combination with 5-azacitidine in patients with myelofibrosis. Blood. 2018;132:352 Results of a phase 2 study of ruxolitinib plus azacitidine in patients with myelofibrosis.
Moyo TK, Palmer J, Huang Y, Oluwole O, Mohan SR, Caza R, Ayers GD, Berry LD, Dudley CV, Dugger L, Miskin HP, Cavers A, Sportelli P, McMahon B, Strickland SA, Mesa RA, Michaelis LC, Savona MR. Resurrecting response to ruxolitinib: a phase I study of ruxolitinib and umbralisib (TGR-1202) in ruxolitinib-experienced myelofibrosis. Haematologica 2018:S133.
Daver NG, Kremyanskaya M, O’Connell C, Dao K, Oh ST, Gerds AT, et al. A phase 2 study of the safety and efficacy of INCB050465, a selective PI3Kδ inhibitor, in combination with ruxolitinib in patients with myelofibrosis. Blood. 2018;132:353.
Gupta V, Harrison CN, Hasselbalch HC, Pieri L, Koschmieder S, Cervantes F, et al. Phase 1b/2 study of the efficacy and safety of sonidegib (LDE225) in combination with ruxolitinib (INC424) in patients with myelofibrosis. Blood. 2015;126:825.
Rampal RK, Verstovsek S, Devlin SM, Stein EM, Kadia TM, Mauro MJ, et al. Safety and efficacy of combined ruxolitinib and thalidomide in patients with myelofibrosis: initial results of a phase ii study. Blood. 2018;132:354.
Komrokji R, Garcia-Manero G, Ades L, Prebet T, Steensma DP, Jurcic JG, et al. Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: a phase 2, dose-ranging trial. Lancet Haematol. 2018;5:e63–72.
Platzbecker U, Germing U, Gotze KS, Kiewe P, Mayer K, Chromik J, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017;18:1338–47.
Mascarenhas J, Li T, Sandy L, Newsom C, Petersen B, Godbold J, et al. Anti-transforming growth factor-beta therapy in patients with myelofibrosis. Leuk Lymphoma. 2014;55:450–2.
Iancu-Rubin C, Mosoyan G, Wang J, Kraus T, Sung V, Hoffman R. Stromal cell-mediated inhibition of erythropoiesis can be attenuated by Sotatercept (ACE-011), an activin receptor type II ligand trap. Exp Hematol. 2013;41:155–66 e17.
Carrancio S, Markovics J, Wong P, Leisten J, Castiglioni P, Groza MC, et al. An activin receptor IIA ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cells and haemoglobinBr J Haematol. 2014;165:870–82.
• Bose P, Daver N, Pemmaraju N, Jabbour EJ, Estrov Z, Pike A, et al. Sotatercept (ACE-011) Alone and in combination with ruxolitinib in patients (pts) with myeloproliferative neoplasm (MPN)-associated myelofibrosis (MF) and anemia. Blood. 2017;130:255 The only available clinical data so far on activin receptor ligand traps in myelofibrosis.
• Fenaux P, Platzbecker U, Mufti GJ, Garcia-Manero G, Buckstein R, Santini V, et al. The Medalist Trial: results of a phase 3, randomized, double-blind, placebo-controlled study of luspatercept to treat anemia in patients with very low-, low-, or intermediate-risk myelodysplastic syndromes (MDS) with ring sideroblasts (RS) who require red blood cell (RBC) transfusions. Blood. 2018;132:1 ASH 2018 plenary session abstract on the MEDALIST trial of luspatercept in patients with lower risk MDS with ring sideroblasts.
Spivak JL. Myeloproliferative neoplasms. N Engl J Med. 2017;376:2168–81.
• Verstovsek S, Manshouri T, Pilling D, Bueso-Ramos CE, Newberry KJ, Prijic S, et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med. 2016;213:1723–40 Evidence that the cells that lead to bone marrow fibrosis in patients with myelofibrosis are monocyte-derived and clonal.
Guglielmelli P, Rotunno G, Pacilli A, Rumi E, Rosti V, Delaini F, et al. Prognostic impact of bone marrow fibrosis in primary myelofibrosis. A study of the AGIMM group on 490 patients. Am J Hematol. 2016;91:918–22.
• Guglielmelli P, Lasho TL, Rotunno G, Mudireddy M, Mannarelli C, Nicolosi M, et al. MIPSS70: Mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2017;36:310–8. https://doi.org/10.1200/JCO2017764886 Description of the MIPSS70 and the MIPSS70-plus, prognostic scoring systems for transplant-age patients with primary myelofibrosis that integrate clinical and genomic information.
• Guglielmelli P, Pacilli A, Rotunno G, Rumi E, Rosti V, Delaini F, et al. Presentation and outcome of patients with 2016 WHO diagnosis of prefibrotic and overt primary myelofibrosis. Blood. 2017;129:3227–36 A large study delineating the differences in presentation and outcome between individuals with pre-fibrotic and overt primary myelofibrosis.
Verstovsek S, Mesa RA, Foltz LM, Gupta V, Mascarenhas JO, Ritchie EK, et al. Phase 2 trial of PRM-151, an anti-fibrotic agent, in patients with myelofibrosis: stage 1 results. Blood. 2014;124:713.
Verstovsek S, Mesa RA, Foltz LM, Gupta V, Mascarenhas JO, Ritchie EK, et al. PRM-151 in myelofibrosis: durable efficacy and safety at 72 weeks. Blood. 2015;126:56.
Verstovsek S, Hasserjian RP, Pozdnyakova O, Veletic I, Mesa RA, Foltz L, et al. PRM-151 in myelofibrosis: efficacy and safety in an open label extension study. Blood. 2018;132:686.
• Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PMH, et al. Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2017;20:785–800.e8 Preclinical work describing the pathogenic role of Gli1+ MSCs in bone marrow fibrosis.
Sasaki K, Gotlib JR, Mesa RA, Newberry KJ, Ravandi F, Cortes JE, et al. Phase II evaluation of IPI-926, an oral Hedgehog inhibitor, in patients with myelofibrosis. Leuk Lymphoma. 2015;56:2092–7.
• Yue L, Bartenstein M, Zhao W, Ho WT, Han Y, Murdun C, et al. Efficacy of ALK5 inhibition in myelofibrosis. JCI Insight. 2017;2:e90932. Preclinical support for targeting the TGF-ß receptor kinase in myelofibrosis.
• Decker M, Martinez-Morentin L, Wang G, Lee Y, Liu Q, Leslie J, et al. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol. 2017;19:677–88 A role for targeting PDGFRA to improve bone marrow fibrosis in primary myelofibrosis?
• Maekawa T, Osawa Y, Izumi T, Nagao S, Takano K, Okada Y, et al. Myeloproliferative leukemia protein activation directly induces fibrocyte differentiation to cause myelofibrosis. Leukemia. 2017;31:2709–16 Preclinical findings showing that circulating SLAMF7high MPLhigh monocytes may be the precursors of fibrocytes that drive bone marrow fibrosis.
Verstovsek S, Talpaz M, Ritchie EK, Wadleigh M, Odenike O, Jamieson C, et al. Phase 1/2 study of NS-018, an oral JAK2 inhibitor, in patients with primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (postPV MF), or post-essential thrombocythemia myelofibrosis (postET MF). Blood. 2016;128:1936.
Bose P, Abou Zahr A, Verstovsek S. Investigational Janus kinase inhibitors in development for myelofibrosis. Expert Opin Investig Drugs. 2017.
Giacomini MM, Hao J, Liang X, Chandrasekhar J, Twelves J, Whitney JA, et al. Interaction of 2,4-diaminopyrimidine-containing drugs including fedratinib and trimethoprim with thiamine transporters. Drug Metab Dispos. 2017;45:76–85.
Zhang Q, Zhang Y, Diamond S, Boer J, Harris JJ, Li Y, et al. The Janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke’s encephalopathy. Drug Metab Dispos. 2014;42:1656–62.
• Harrison CN, Mesa RA, Jamieson C, Hood J, Bykowski J, Zuccoli G, et al. Case series of potential Wernicke’s encephalopathy in patients treated with fedratinib. Blood. 2017;130:4197 Analysis of the putative cases of Wernicke’s encephalopathy in fedratinib-treated patients.
• Pardanani A, Harrison C, Cortes JE, Cervantes F, Mesa RA, Milligan D, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1:643–51 The JAKARTA phase 3 placebo-controlled trial of fedratinib in myelofibrosis.
• Harrison CN, Schaap N, Vannucchi AM, Kiladjian JJ, Tiu RV, Zachee P, et al. Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4:e317–24 The single-arm, open-label, phase 2 JAKARTA-2 trial of fedratinib in ruxolitinib-exposed patients with myelofibrosis.
Singer JW, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, Verstovsek S. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol. 2016;8:11–9.
• Mesa RA, Vannucchi AM, Mead A, Egyed M, Szoke A, Suvorov A, et al. Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial. Lancet Haematol. 2017;4:e225–36 The phase 3 PERSIST-1 trial of pacritinib versus best available therapy in myelofibrosis.
• Mascarenhas J, Hoffman R, Talpaz M, Gerds AT, Stein B, Gupta V, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4:652–9 The phase 3 PERSIST-2 trial testing two doses of pacritinib against best available therapy in myelofibrosis.
CTI BioPharma provides update on clinical hold of investigational agent pacritinib and new drug application in U.S. 2016.
CTI BioPharma announces removal of full clinical hold on pacritinib. 2017;2017.
Pardanani A, Gotlib JR, Gupta V, Roberts AW, Wadleigh M, Sirhan S, et al. Update on the long-term efficacy and safety of momelotinib, a JAK1 and JAK2 inhibitor, for the treatment of myelofibrosis. Blood. 2013;122:108.
Gupta V, Mesa RA, Deininger MW, Rivera CE, Sirhan S, Brachmann CB, et al. A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica. 2016;102:94–102.
• Asshoff M, Petzer V, Warr MR, Haschka D, Tymoszuk P, Demetz E, et al. Momelotinib inhibits ACVR1/ALK2, decreases hepcidin production and ameliorates anemia of chronic disease in rodents. Blood. 2017;129:1823–30 Mechanistic study that could explain how momelotinib improves anemia.
• Mesa RA, Kiladjian JJ, Catalano JV, Devos T, Egyed M, Hellmann A, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naive patients with myelofibrosis. J Clin Oncol. 2017;35:3844–50. https://doi.org/10.1200/JCO2017734418 The head to head phase 3 trial comparing momelotinib to ruxolitinib in myelofibrosis.
• Harrison CN, Vannucchi AM, Platzbecker U, Cervantes F, Gupta V, Lavie D, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2017;5:e73–81 Phase 3 trial of momelotinib versus best available therapy in ruxolitinib-treated patients with myelofibrosis.
Abdelrahman RA, Begna KH, Al-Kali A, Hogan WJ, Litzow MR, Pardanani A, et al. Momelotinib treatment-emergent neuropathy: prevalence, risk factors and outcome in 100 patients with myelofibrosis. Br J Haematol. 2015;169:77–80.
Tefferi A, Barraco D, Lasho TL, Shah S, Begna KH, Al-Kali A, et al. Momelotinib therapy for myelofibrosis: a 7-year follow-up. Blood Cancer J. 2018;8:29. https://doi.org/10.1038/s41408-018-0067-6.
Perez C, Pascual M, Martin-Subero JI, Bellosillo B, Segura V, Delabesse E, et al. Aberrant DNA methylation profile of chronic and transformed classic Philadelphia-negative myeloproliferative neoplasms. Haematologica. 2013;98:1414–20.
Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–26,734.
Evrot E, Ebel N, Romanet V, Roelli C, Andraos R, Qian Z, et al. JAK1/2 and Pan-deacetylase inhibitor combination therapy yields improved efficacy in preclinical mouse models of JAK2V617F-driven disease. Clin Cancer Res. 2013;19:6230–41.
Mascarenhas J, Lu M, Li T, Petersen B, Hochman T, Najfeld V, et al. A phase I study of panobinostat (LBH589) in patients with primary myelofibrosis (PMF) and post-polycythaemia vera/essential thrombocythaemia myelofibrosis (post-PV/ET MF). Br J Haematol. 2013;161:68–75.
Mascarenhas J, Sandy L, Lu M, Yoon J, Petersen B, Zhang D, et al. A phase II study of panobinostat in patients with primary myelofibrosis (PMF) and post-polycythemia vera/essential thrombocythemia myelofibrosis (post-PV/ET MF). Leuk Res. 2016;53:13–9.
Quintas-Cardama A, Kantarjian H, Estrov Z, Borthakur G, Cortes J, Verstovsek S. Therapy with the histone deacetylase inhibitor pracinostat for patients with myelofibrosis. Leuk Res. 2012;36:1124–7.
Verstovsek S, Fiskus W, Manshouri T, Bhalla KN. Targeting cistrome and dysregulated transcriptome of post-MPN sAML. Oncotarget. 2017;8:93301–93,302.
• Saenz DT, Fiskus W, Manshouri T, Rajapakshe K, Krieger S, Sun B, et al. BET protein bromodomain inhibitor-based combinations are highly active against post-myeloproliferative neoplasm secondary AML cells. Leukemia. 2016;31:678–87 Preclinical evidence of synergism between ruxolitinib and bromodomain inhibitors against post-MPN AML.
• Kleppe M, Koche R, Zou L, van Galen P, Hill CE, Dong L, et al. Dual targeting of oncogenic activation and inflammatory signaling increases therapeutic efficacy in myeloproliferative neoplasms. Cancer Cell. 2018;33:29–43.e7 Preclinical evidence of synergism between ruxolitinib and bromodomain inhibitors in MPN models.
Khan I, Huang Z, Wen Q, Stankiewicz MJ, Gilles L, Goldenson B, et al. AKT is a therapeutic target in myeloproliferative neoplasms. Leukemia. 2013;27:1882–90.
Bogani C, Bartalucci N, Martinelli S, Tozzi L, Guglielmelli P, Bosi A, et al. Associazione Italiana per la Ricerca sul Cancro AGIMM Gruppo Italiano Malattie Mieloproliferative. mTOR inhibitors alone and in combination with JAK2 inhibitors effectively inhibit cells of myeloproliferative neoplasms. PLoS One. 2013;8:e54826.
Fiskus W, Verstovsek S, Manshouri T, Smith JE, Peth K, Abhyankar S, et al. Dual PI3K/AKT/mTOR inhibitor BEZ235 synergistically enhances the activity of JAK2 inhibitor against cultured and primary human myeloproliferative neoplasm cells. Mol Cancer Ther. 2013;12:577–88.
Mascarenhas J, Komrokji RS, Cavo M, Martino B, Niederwieser D, Reiter A, et al. Imetelstat is effective treatment for patients with intermediate-2 or high-risk myelofibrosis who have relapsed on or are refractory to Janus kinase inhibitor therapy: results of a phase 2 randomized study of two dose levels. Blood. 2018;132:685.
Pemmaraju N, Carter BZ, Kantarjian HM, Cortes JE, Kadia TM, Garcia-Manero G, et al. LCL161, an oral Smac mimetic/IAP antagonist for patients with myelofibrosis (MF): novel translational findings among long-term responders in a phase 2 clinical trial. Blood. 2018;132:687.
Guglielmelli P, Barosi G, Rambaldi A, Marchioli R, Masciulli A, Tozzi L, et al. AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM) investigators. Safety and efficacy of everolimus, a mTOR inhibitor, as single agent in a phase 1/2 study in patients with myelofibrosis. Blood. 2011;118:2069–76.
Mascarenhas JO, Talpaz M, Gupta V, Foltz LM, Savona MR, Paquette R, et al. Primary analysis of a phase II open-label trial of INCB039110, a selective JAK1 inhibitor, in patients with myelofibrosis. Haematologica. 2016;102:327–35.
Gangat N, Stein BL, Marinaccio C, Swords R, Watts JM, Gurbuxani S, et al. Alisertib (MLN8237), an oral selective inhibitor of aurora kinase a, has clinical activity and restores GATA1 expression in patients with myelofibrosis. Blood. 2018;132:688.
Gerds AT, Tauchi T, Ritchie EK, Deininger MW, Jamieson CH, Mesa R, et al. Phase I/II trial of glasdegib in heavily pre-treated patients with primary or secondary myelofibrosis. Blood. 2017;130:258.
• Klein C, Zwick A, Kissel S, Forster CU, Pfeifer D, Follo M, et al. Ptch2 loss drives myeloproliferation and myeloproliferative neoplasm progression. J Exp Med. 2016;213:273–90 Preclinical studies demonstrating the importance of the hedgehog pathway in MPN biology.
Bhagwat N, Keller MD, Rampal RK, Shank K, de Stanchina E, Rose K, et al. Improved efficacy of combination of JAK2 and hedgehog inhibitors in myelofibrosis. Blood. 2013;122:666.
Couban S, Benevolo G, Donnellan W, Cultrera J, Koschmieder S, Verstovsek S, et al. Phase 1b results of a study to assess the efficacy and safety of vismodegib in combination with ruxolitinib in patients with intermediate- or high-risk myelofibrosis. Blood. 2017;130:4179.
Koppikar P, Bhagwat N, Kilpivaara O, Manshouri T, Adli M, Hricik T, et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature. 2012;489:155–9.
Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R, et al. Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms. Blood. 2014;123:2075–83.
Fiskus W, Verstovsek S, Manshouri T, Rao R, Balusu R, Venkannagari S, et al. Heat shock protein 90 inhibitor is synergistic with JAK2 inhibitor and overcomes resistance to JAK2-TKI in human myeloproliferative neoplasm cells. Clin Cancer Res. 2011;17:7347–58.
Hobbs GS, Hanasoge Somasundara AV, Kleppe M, Litvin R, Arcila M, Ahn J, et al. Hsp90 inhibition disrupts JAK-STAT signaling and leads to reductions in splenomegaly in patients with MPNs. Haematologica. 2017;103:e5–9.
Waibel M, Solomon VS, Knight DA, Ralli RA, Kim SK, Banks KM, et al. Combined targeting of JAK2 and Bcl-2/Bcl-xL to cure mutant JAK2-driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell Rep. 2013;5:1047–59.
Fleischman AG, Aichberger KJ, Luty SB, Bumm TG, Petersen CL, Doratotaj S, et al. TNFalpha facilitates clonal expansion of JAK2V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118:6392–8.
Heaton WL, Senina AV, Pomicter AD, Salama ME, Clair PM, Yan D, et al. Autocrine Tnf signaling favors malignant cells in myelofibrosis in a Tnfr2-dependent fashion. Leukemia. 2018;32:2399–411.
Rampal R, Pinzon-Ortiz M, Varshini HSA, Levine RL, Cao A. Synergistic therapeutic efficacy of combined JAK1/2, Pan-PIM, and CDK4/6 inhibition in myeloproliferative neoplasms. Blood. 2016;128:634.
Huang SM, Wang A, Greco R, Li Z, Barberis C, Tabart M, et al. Combination of PIM and JAK2 inhibitors synergistically suppresses MPN cell proliferation and overcomes drug resistance. Oncotarget. 2014;5:3362–74.
Mazzacurati L, Lambert QT, Pradhan A, Griner LN, Huszar D, Reuther GW. The PIM inhibitor AZD1208 synergizes with ruxolitinib to induce apoptosis of ruxolitinib sensitive and resistant JAK2-V617F-driven cells and inhibit colony formation of primary MPN cells. Oncotarget. 2015;6:40141–40,157.
Nath D, Dutta A, Yang Y, Whatcott C, Warner SL, Mohi G. The PIM kinase inhibitor TP-3654 in combination with ruxolitinib exhibits marked improvement of myelofibrosis in murine models. Blood. 2018;132:54.
• Gilles L, Arslan AD, Marinaccio C, Wen QJ, Arya P, McNulty M, et al. Downregulation of GATA1 drives impaired hematopoiesis in primary myelofibrosis. J Clin Invest. 2017;127:1316–20 Important insights into megakaryocyte biology in primary myelofibrosis.
• Wen QJ, Yang Q, Goldenson B, Malinge S, Lasho T, Schneider RK, et al. Targeting megakaryocytic induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat Med. 2015;21:1473–80 A novel strategy to possibly ameliorate bone marrow fibrosis in myelofibrosis.
Pratz KW, Koh BD, Patel AG, Flatten KS, Poh W, Herman JG, et al. Poly (ADP-Ribose) polymerase inhibitor hypersensitivity in aggressive myeloproliferative neoplasms. Clin Cancer Res. 2016;22:3894–902.
• Nieborowska-Skorska M, Maifrede S, Dasgupta Y, Sullivan K, Flis S, Le BV, et al. Ruxolitinib-induced defects in DNA repair cause sensitivity to PARP inhibitors in myeloproliferative neoplasms. Blood. 2017;130:2848–59 Preclinical paper describing synergism between ruxolitinib and PARP inhibitors.
Pratz KW, Rudek MA, Gojo I, Litzow MR, McDevitt MA, Ji J, et al. A phase I study of topotecan, carboplatin and the PARP inhibitor veliparib in acute leukemias, aggressive myeloproliferative neoplasms, and chronic myelomonocytic leukemia. Clin Cancer Res. 2017;23:899–907.
Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796–800.
Swords RT, Coutre S, Maris MB, Zeidner JF, Foran JM, Cruz J, et al. Pevonedistat, a first-in-class NEDD8-activating enzyme (NAE) inhibitor, combined with azacitidine, in patients with AML. Blood. 2018;131:1415–24.
Park SO, Wamsley HL, Bae K, Hu Z, Li X, Choe SW, et al. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8:e59675.
Kleppe M, Spitzer MH, Li S, Hill CE, Dong L, Papalexi E, et al. Jak1 integrates cytokine sensing to regulate hematopoietic stem cell function and stress hematopoiesis. Cell Stem Cell. 2017;21:489–501.e7.
• Tefferi A, Lasho TL, Begna KH, Patnaik MM, Zblewski DL, Finke CM, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med. 2015;373:908–19 Original report of the efficacy of imetelstat in myelofibrosis.
Janssen. Janssen elects not to continue agreement with Geron for imetelstat. 2018;2018. https://www.janssen.com/janssen-elects-not-continue-agreement-geronimetelstat/. Accessed 27 Sept 2018
Nakatake M, Monte-Mor B, Debili N, Casadevall N, Ribrag V, Solary E, et al. JAK2(V617F) negatively regulates p53 stabilization by enhancing MDM2 via La expression in myeloproliferative neoplasms. Oncogene. 2012;31:1323–33.
Mascarenhas J, Lu M, Virtgaym E, Kosiorek H, Stal M, Sandy L, et al. Open label phase I study of single agent oral RG7388 (idasanutlin) in patients with polycythemia vera and essential thrombocythemia. Blood. 2017;130:254.
Meyer SC, Ghosh N, Stivala S, Baerenwaldt A, Hao-Shen H, Dirnhofer S, et al. Targeting cell non-autonomous MAPK activation as a novel therapeutic strategy in myeloproliferative neoplasms. Blood. 2017;130:381.
Nguyen TK, Tata P, Brooks S, Jena N, Morse SJ, Liu Z, et al. The MEK/ERK inhibitor trametinib reduces fibrosis in a transduction-transplantation model of mutated calreticulin. Blood. 2016;128:635.
Yan D, Tantravahi SK, Pomicter AD, Senina A, Gantz KC, Redwine HM, et al. Selective inhibition of nuclear cytoplasmic transport as a new treatment paradigm in myelofibrosis. Blood. 2016;128:636.
• Elf S, Abdelfattah NS, Baral AJ, Beeson D, Rivera JF, Ko A, et al. Defining the requirements for the pathogenic interaction between mutant calreticulin and MPL in MPN. Blood. 2018;131:782–6 Mechanistic insights into how mutant calreticulin is an oncogenic driver in MPN.
Holmstrom MO, Riley CH, Svane IM, Hasselbalch HC, Andersen MH. The CALR exon 9 mutations are shared neoantigens in patients with CALR mutant chronic myeloproliferative neoplasms. Leukemia. 2016;30:2413–6.
• Holmstrom MO, Martinenaite E, Ahmad SM, Met O, Friese C, Kjaer L, et al. The calreticulin (CALR) exon 9 mutations are promising targets for cancer immune therapy. Leukemia. 2018;32:429–37 Introduction to the concept of targeting mutant calreticulin using immunotherapeutic approaches.
Silver RT, Barel AC, Lascu E, Ritchie EK, Roboz GJ, Christos PJ, et al. The effect of initial molecular profile on response to recombinant interferon-alpha (rIFNalpha) treatment in early myelofibrosis. Cancer. 2017;123:2680–7.
Gisslinger H, Gisslinger B, Schalling M, Krejcy K, Widmann RS, Kralovics R, et al. Effect of ropeginterferon alfa-2b in prefibrotic primary myelofibrosis. Blood. 2018;132:3029.
Kiladjian J, Soret-Dulphy J, Resche-Rigon M, Boyer-Perrard F, Barraco F, Rolland-Neyret V, et al. Ruxopeg, a multi-center Bayesian phase 1/2 adaptive randomized trial of the combination of ruxolitinib and pegylated interferon alpha 2a in patients with myeloproliferative neoplasm (MPN)-associated myelofibrosis. Blood. 2018;132:581.
Funding
This work was supported, in part, by the MD Anderson Cancer Center support grant, P30 CA016672, from the National Institutes of Health (National Cancer Institute).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Prithviraj Bose has received research funding from Incyte Corporation, Celgene, Blueprint Medicines, Constellation Pharmaceuticals, Kartos Therapeutics, CTI BioPharma, Astellas, and Pfizer, and has received compensation from Incyte Corporation, Celgene, and Blueprint Medicines for service as a consultant.
Mansour Alfayez declares that he has no conflict of interest.
Srdan Verstovsek has received research support for the conduct of clinical studies from Incyte Corporation, Roche, NS Pharma, Celgene, Gilead, Promedior, CTI BioPharma, Genentech, Blueprint Medicines, and Novartis, and has received compensation from Constellation Pharmaceuticals, Pragmatist, Sierra, Incyte Corporation, Novartis, and Celgene for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Bose, P., Alfayez, M. & Verstovsek, S. New Concepts of Treatment for Patients with Myelofibrosis. Curr. Treat. Options in Oncol. 20, 5 (2019). https://doi.org/10.1007/s11864-019-0604-y
Published:
DOI: https://doi.org/10.1007/s11864-019-0604-y