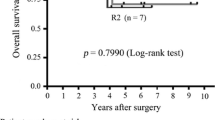
Opinion statement
Gastrointestinal stromal tumors (GISTs) are the most common sarcomas and mesenchymal neoplasms of the gastrointestinal tract. Macroscopically complete (R0/R1) resection is the standard treatment for localized resectable GIST with adjuvant imatinib therapy recommended for patients with intermediate or high-risk disease. In patients with advanced unresectable or metastatic GIST, imatinib has significantly improved outcomes. However, while most patients achieve partial response (PR) or stable disease (SD) on imatinib (with maximal response typically seen by 6 months on treatment), approximately half will develop secondary resistance by 2 years. Available data suggest that cytoreductive surgery may be considered in patients with metastatic GIST who respond to imatinib, particularly if a R0/R1 resection is achieved. The benefit of surgery in patients with focal tumor progression on imatinib is unclear, but may be considered. Patients with multifocal progression undergoing surgery generally have poor outcomes. Thus, surgery should be considered in patients with metastatic GIST whose disease responds to imatinib with a goal of performing R0/R1 resection. Optimal timing of surgery is unclear but should be considered between 6 months and 2 years after starting imatinib. Although surgery in patients with metastatic GIST treated with sunitinib is feasible, incomplete resections are common, complication rates are high, and survival benefit is unclear. Therefore, a careful multidisciplinary consultation is required to determine optimal treatment options on a case-by-case basis. Finally, patients with metastatic GIST should resume tyrosine kinase inhibitor treatment postoperatively.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Quek R, George S. Gastrointestinal stromal tumor: a clinical overview. Hematol Oncol Clin North Am. 2009;23:69–78.
Maki RG et al. Key issues in the clinical management of gastrointestinal stromal tumors : an expert discussion. Oncologist. 2015;20:823–30.
Gold JS, Dematteo RP. Combined surgical and molecular therapy: the gastrointestinal stromal tumor model. Ann Surg. 2006;244:176–84.
Nilsson B et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era—a population-based study in western Sweden. Cancer. 2005;103:821–9.
Hirota S et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80.
Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20:1692–703.
Heinrich MC et al. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–32.
Tuveson DA et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001;20:5054–8.
Gronchi A, Raut CP. The combination of surgery and Imatinib in GIST: a reality for localized tumors at high risk, an open issue for metastatic ones. Ann Surg Oncol. 2012;19:1370. Overview of combination imatinib treatment and surgery in patients with localized GIST with high risk of recurrence and metastatic GIST.
DeMatteo RP et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer. 2008;112:608–15.
Joensuu H et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–74. Using pooled individual patient data from ten population-based series from nine countries, these authors compared the performance of the most commonly used risk-stratification schemes and developed a new prognostic model for patients with operable GIST.
McAuliffe JC et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol. 2009;16:910–9.
Hohenberger, P. et al. Neoadjuvant treatment of locally advanced GIST: results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. J Clin Oncol. 30, (Suppl; abstr 10031) (2012).
Wang D et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of radiation therapy oncology group 0132. Ann Surg Oncol. 2012;19:1074–80.
Eisenberg BL et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42–7.
Joensuu H. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor. JAMA. 2012;307:1265–72. Randomized phase III study in which patients received 12 months or 36 months of adjuvant imatinib and which demonstrated improved RFS and OS with 36 months of imatinib among patients with operable GIST at high risk of recurrence.
Joensuu H et al. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer. 2014;120:2325–33.
Corless CL et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563–70.
DeMatteo RP et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor. Ann Surg. 2013;258:422–9. Phase II intergroup trial led by the American College of Surgeons Oncology Group (NCT00025246) demonstrating increased OS among patients with primary GIST at high risk of recurrence who receive adjuvant imatinib for 1 year compared to historical controls.
McCarter MD et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012;215:53–9.
Casali, P. et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): The EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol Clin Oncol 31, (suppl; abstr 10500) (2013).
Raut, C. et al. Adjuvant imatinib (IM) for patients (pts) with primary gastrointestinal stromal tumor (GIST) at significant risk of recurrence: PERSIST-5 planned 3-year interim analysis. J Clin Oncol 33, (suppl; abstr 10537) (2015).
Vadakara J, von Mehren M. Gastrointestinal stromal tumors. Management of metastatic disease and emerging therapies. Hematol Oncol Clin North Am. 2013;27:905–20.
Tielen R et al. Surgery after treatment with imatinib and/or sunitinib in patients with metastasized gastrointestinal stromal tumors: is it worthwhile? World J Surg Oncol. 2012;10:111.
Demetri GD, von Mehren M, Blanke CD, Abbeele. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. NEJM. 2002;347:472–80.
Haller F et al. Surgical management after neoadjuvant imatinib therapy in gastrointestinal stromal tumours (GISTs) with respect to imatinib resistance caused by secondary KIT mutations. Ann Surg Oncol. 2007;14:526–32.
An HJ et al. The effects of surgical cytoreduction prior to imatinib therapy on the prognosis of patients with advanced GIST. Ann Surg Oncol. 2013;20:4212–8.
Bonvalot S et al. Impact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib era. Ann Surg Oncol. 2006;13:1596–603.
Scaife CL et al. Is there a role for surgery in patients with ‘unresectable’ cKIT+ gastrointestinal stromal tumors treated with imatinib mesylate? Am J Surg. 2003;186:665–9.
Andtbacka RHI et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol. 2007;14:14–24.
Bauer S et al. Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer. 2005;117:316–25. Retrospective analysis of 239 patients with metastatic GIST who received imatinib and underwent metastasectomy demonstrating that long-term survival can be achieved in patients in whom surgical complete resection can be achieved while incomplete resection failed to prolong survival.
Gold JS et al. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol. 2007;14:134–42.
Raut CP et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006;24:2325–31.
Zaydfudim V, Okuno SH, Que FG, Nagorney DM, Donohue JH. Role of operative therapy in treatment of metastatic gastrointestinal stromal tumors. J Surg Res. 2012;177:248–54.
Bischof DA et al. Surgical management of advanced gastrointestinal stromal tumors: an international multi-institutional analysis of 158 patients. J Am Coll Surg. 2014;219:439–49.
Rutkowski P et al. Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol. 2006;93:304–11.
Mussi C et al. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol. 2010;21:403–8.
Gronchi A et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg. 2007;245:341–6.
Sym SJ et al. Surgical intervention following imatinib treatment in patients with advanced gastrointestinal stromal tumors (GISTs). J Surg Oncol. 2008;98:27–33.
DeMatteo RP et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg. 2007;245:347–52.
Bauer S et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib—analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol. 2014;40:412–9.
Du CY et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer. 2014;50:1772–8. Patients with recurrent/metastatic GISTs receiving imatinib were randomized to surgery for residual disease or imatinib alone (ChiCTR-TRC-00000244). Trial was closed early due to poor accrual however among the 41 patients enrolled, those patients on imatinib who underwent resection of residual disease had longer PFS and OS.
Rubió-Casadevall J et al. Role of surgery in patients with recurrent, metastatic, or unresectable locally advanced gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol. 2015;22:2948–57. Multi-institutional analysis of 171 patients with recurrent unresectable locally advanced or metastatic GIST treated with imatinib only vs imatinib and metastasectomy demonstrating increased OS with metastasectomy among imatinib responders.
Barnes G et al. A review of the surgical management of metastatic gastrointestinal stromal tumours (GISTs) on imatinib mesylate (Glivec). Int J Surg. 2005;3:206–12.
Demetri GD et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38.
Raut CP et al. Cytoreductive surgery in patients with metastatic gastrointestinal stromal tumor treated with sunitinib malate. Ann Surg Oncol. 2010;17:407–15.
Le Cesne A et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11:942–9. Phase III randomized trial demonstrating imatinib interruption after 3 years in responders results in high risk of rapid progression among patients with advanced GIST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Sarcoma
Rights and permissions
About this article
Cite this article
Keung, E.Z., Fairweather, M. & Raut, C.P. The Role of Surgery in Metastatic Gastrointestinal Stromal Tumors. Curr. Treat. Options in Oncol. 17, 8 (2016). https://doi.org/10.1007/s11864-015-0384-y
Published:
DOI: https://doi.org/10.1007/s11864-015-0384-y