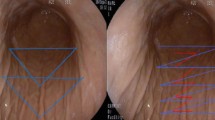
Abstract
Introduction
Endoscopic gastroplasty and gastric volume reduction techniques have been shown to achieve significant weight loss and improvement in comorbid conditions. The objective of this study is to assess the feasibility and safety of a novel fully automated, operator-independent endoscopic suturing system (EndoZip™) for minimally invasive treatment of obesity.
Design
Single-center pilot feasibility study.
Patients
Eleven patients with a body mass index (BMI) of 30 to 40 kg/m2 with or without obesity-associated comorbidity.
Interventions
Gastric volume reduction with EndoZip™ system.
Main Outcome Measurements
Primary outcome was to assess the technical feasibility and safety. The secondary outcome was to determine %total body weight loss (TBWL) and %excess weight loss (EWL) at 6 months.
Results
The mean ± SD age was 42.7 ± 5.6 years, and the mean ± SD BMI was 36.9 ± 2.8 kg/m2. A majority (64%) were men. The procedure was technically successful (100%) in all patients. A median of 3 (range, 2–4) full-thickness sutures were placed, and the mean procedure time was 54.6 ± 23.9 (23–100) min. No immediate complications occurred, and all were discharged in 24 h. One patient developed respiratory infection 3 days after the procedure and required hospitalization. The infection was mild and resolved with antibiotic treatment. At 6-month follow-up, the mean ± SD TBWL, %TBWL, and %EWL were 17.8 ± 6.7 kg, 16.2 ± 6.0%, and 54.3 ± 28.4%, respectively (p < 0.001).
Limitations
Limited number of patients.
Conclusion
Our first-in-human study showed that the Endozip™ device could be safely used for the treatment of obesity. The early weight loss results are promising. An extended feasibility study on a larger sample size is being planned (Clinicaltrials.gov. NCT03472196).




Similar content being viewed by others
Abbreviations
- TBWL:
-
Total body weight loss
- EWL:
-
Excess weight loss
- BMI:
-
Body mass index
- IGB:
-
Intragastric balloon
- ESG:
-
Endoscopic sutured gastroplasty
- SD:
-
Standard deviation
References
Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78:530–5.
Wallstabe I, Oberaender N, Weimann A, et al. Endoscopic sleeve gastroplasty using the novel Endomina device for morbidly obese patients. Endoscopy. 2018;50:E327–8.
Lopez-Nava G, Galvão MP, da Bautista-Castaño I, et al. Endoscopic sleeve gastroplasty for the treatment of obesity. Endoscopy. 2015;47:449–52.
Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 248 patients with 24 months follow-up. Obes Surg. 2017;27:2649–55.
Alqahtani A, Al-Darwish A, Mahmoud AE, et al. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest Endosc. 2019;89:1132–8.
Li P, Ma B, Gong S, et al. Efficacy and safety of endoscopic sleeve gastroplasty for obesity patients: a meta-analysis. Surg Endosc. 2019 Jun;24:1–8. https://doi.org/10.1007/s00464-019-06889-6.
Cheskin LJ, Hill C, Adam A, et al. Endoscopic sleeve gastroplasty versus high-intensity diet and lifestyle therapy: a case-matched study. Gastrointest Endosc. 2019; https://doi.org/10.1016/j.gie.2019.09.029.
Fayad L, Cheskin LJ, Adam A, et al. Endoscopic sleeve gastroplasty versus intragastric balloon insertion: efficacy, durability, and safety. Endoscopy. 2019;51:532–9.
Sharaiha RZ, Kumta NA, Saumoy M, et al. Endoscopic sleeve gastroplasty significantly reduces body mass index and metabolic complications in obese patients. Clin Gastroenterol Hepatol. 2017;15:504–10.
Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol. 2017;15:37–43.e1.
Saumoy M, Schneider Y, Zhou XK, et al. A single-operator learning curve analysis for the endoscopic sleeve gastroplasty. Gastrointest Endosc. 2018;87:442–7.
Hill C, El Zein M, Agnihotri A, et al. Endoscopic sleeve gastroplasty: the learning curve. Endosc Int Open. 2017;5:E900–4.
Shahnazarian V, Ramai D, Sarkar A. Endoscopic bariatric therapies for treating obesity: a learning curve for gastroenterologists. Transl Gastroenterol Hepatol. 2019;18(4):16.
Movitz BR, Lutfi RE. Endoscopic sleeve gastroplasty: are we burning bridges? Surg Obes Relat Dis. 2017;13:2056–8.
Jirapinyo P, Thompson CC. Training in bariatric and metabolic endoscopic therapies. Clin Endosc. 2018;51:430–8.
Turkeltaub JA, Edmundowicz SA. Endoscopic bariatric therapies: intragastric balloons, tissue apposition, and aspiration therapy. Curr Treat Options Gastroenterol. 2019;17:187–201.
Goyal D, Singh VK, Amateau SK, et al. Evolution of endoscopic bariatric devices: from development to practice. Am J Gastroenterol. 2019;114:679–83.
Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic bariatric and metabolic therapies: new and emerging technologies. Gastroenterology. 2017;152:1791–801.
Machytka E, Bužga M, Zonca P, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc. 2017;86:904–12.
Grupo Colaborativo de la Sociedad Española de Nutrición Comunitaria (SENC, Aranceta Bartrina J, Arija Val V et al. Dietary guidelines for the Spanish population (SENC, December 2016); the new graphic icon of healthy nutrition. Nutr Hosp. 2016; 33: 1–48.
ASGE Bariatric Endoscopy Task Force and ASGE Technology Committee, Abu Dayyeh BK, Kumar N, et al. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425–38.e5.
Aurora A, Khaitan L, Saber A. Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc. 2012;26:1509–15.
Elms L, Moon R, Varnadore S, et al. Causes of small bowel obstruction after roux-en-Y gastric bypass: a review of 2,395 cases at a single institution. Surg Endosc. 2014;28:1624–8.
Jirapinyo P, Thompson CC. Endoscopic bariatric and metabolic therapies: surgical analogues and mechanisms of action. Clin Gastroenterol Hepatol. 2017;15:619–30.
Hajifathalian K et al. Long-term follow up and outcomes after endoscopic sleeve gastroplasty for treatment of obesity (5 year data). Gastrointest Endosc. 89(6):–AB58.
Alqahtani AR, Elahmedi M, Alqahtani YA, et al. Laparoscopic sleeve gastrectomy after endoscopic sleeve gastroplasty: technical aspects and short-term outcomes. Obes Surg. 2019. Obes Surg. 2019;29:3547–52.
Sullivan S, Swain JM, Woodman G. Randomized sham-controlled trial evaluating efficacy and safety of endoscopic gastric plication for primary obesity: the ESSENTIAL trial. Obesity (Silver Spring). 2017;25:294–301.
Funding
The study was sponsored by Nitinotes Surgical Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Gontrand Lopez-Nava is a consultant for Nitinotes Surgical, Apollo Endosurgery, and USGI Medical. Dr. Barham Abu Dayyeh is a consultant for Boston Scientific, Metamodix, BFKW, USGI Medical and Nitinotes Surgical. All other authors have no disclosure.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethics Approval
The study was conducted following the good clinical practice guidelines and adhered to the recommendation of the declaration of Helsinki. The institutional review board approved the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopez-Nava, G., Asokkumar, R., Rull, A. et al. Safety and Feasibility of a Novel Endoscopic Suturing Device (EndoZip TM) for Treatment of Obesity: First-in-Human Study. OBES SURG 30, 1696–1703 (2020). https://doi.org/10.1007/s11695-019-04370-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04370-w