Abstract
Background
Intragastric balloons (IGBs) have demonstrated efficacy; however, the percent of “responders” (> 25% estimated weight loss (EWL) or > 10% total body weight loss (TBWL)—as suggested by FDA) have been less reported. The Spatz3 adjustable intragastric balloon (AIGB) extends implantation to 1 year, decreases balloon volume for intolerance, and increases volume for diminishing effect.
Aim
The aim of this study is to determine the efficacy/responder rate of the Spatz3 AIGB.
Methods
Implantations of Spatz3 in 165 consecutive patients (pts) in 2 centers were retrospectively reviewed. Mean BMI is 35.7, mean weight (wt) 99.1 kg, and mean balloon volume 495 ml (400-600 ml). Balloon volume adjustments were offered for intolerance and for wt loss plateau.
Results
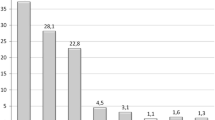
In total, 165 pts were implanted yielding mean wt loss of 16.3 kg, 16.4% TBWL, and 67.4% EWL. Response (> 25% EWL; 10% TBWL) was achieved in 146/165 (88.5%) of patients. Response rates differed for 136 pts with BMI < 40 (91.2%) and 29 pts with BMI > 40 (69%). Down adjustments in 20 patients (mean − 150 ml) allowed 16/20 (80%) to continue IGB therapy. Up adjustments in 64 patients (mean 5.4 months; mean + 260 ml) yielded additional mean wt loss of 5.7 kg. One gastric perforation (0.6%) occurred in a patient who experienced abdominal pain for 2 weeks. Five patients with small ulcers did not require balloon extraction.
Conclusions
(1) Within the limitations of a retrospective review, the Spatz3 balloon appears to be an effective wt loss balloon with better response rates in BMI < 40. (2) Up adjustments yielded a mean 5.7 kg extra wt loss. (3) Down adjustments alleviated early intolerance in 80% of patients. (4) These two adjustment functions may be instrumental in yielding a responder rate of 88.5%.
Similar content being viewed by others
References
Bonazzi P, Petrelli MD, Lorenzini I, et al. Gastric emptying and intragastric balloon in obese patients. Eur Rev Med Pharmacol Sci. 2005;9(5 Suppl 1):15–21.
Totte E, Hendrickx L, Pauwels M, et al. Wt reduction by means of an intragastric device; experience with BIB. Obes Surg. 2001;11(4):519–23.
Courcoulas A et al. Intragastric balloon as an adjunct to lifestyle intervention:a randomized controlled trial. Int J Obes. 2017;41:427–33.
Mathus-Vliegen EM. Intragastric balloon treatment for obesity: what does it really offer? Dig Dis. 2008;26(1):40–4. Review
Imaz I, Martínez-Cervell C, García-Alvarez EE, et al. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18(7):841–6. Review
FDA SSED Reshape Duo Balloon. PMA P140012: FDA summary of safety and effectiveness data. July 28, 2015.
FDA SSED Orbera Balloon. PMA P140008: FDA summary of safety and effectiveness data. August 5, 2015.
Machytka E, Klvana P, Kornbluth A, et al. Adjustable intragastric balloons: a 12-month pilot trial in endoscopic wt loss management. Obes Surg. 2011 Oct;21(10):1499–507.
Brooks J, Srivastava ED, Mathus-Vliegen EMH. One year adjustable intragastric balloons: results in 73 consecutive patients in the UK. Obes Surg. 2014 May;24(5):813–9.
Machytka E, Brooks J, Buzga M and Mason J (2014) One year adjustable intragastric balloon: safety and efficacy of the Spatz3 adjustable balloons [v1; http://f1000r.es/471] F1000Research 2014, 3:203 (doi: 10.12688/f1000research.5099.1)
Mion F, Napoléon B, Roman S, et al. Effects of intragastric balloon on gastric emptying and plasma ghrelin levels in non-morbid obese patients. Obes Surg. 2005;15(4):510–6.
Evans JD, Scott MH. Intragastric balloon in the treatment of patients with morbid obesity. Br J Surg. 2001;88(9):1245–8.
Sallet JA, Marchesini JB, Paiva DS, et al. Brazilian multicenter study of the intragastric balloon. Obes Surg. 2004;14(7):991–8.
Genco A, Cipriano M, Bacci V, et al. Bioenterics intragastric balloon (BIB): a short-term, double-blind, randomized, controlled, crossover study on wt reduction in morbidly obese patients. Int J Obes. 2006;30(1):129–33.
Doldi SB, Micheletto G, Perrini MN, et al. IGB; another option for treatment of obesity and morbid obesity. Hepato-Gastroenterology. 2004;51(55):294–7.
Roman S, Napoleon B, Mion F, et al. IGB for non-morbid obesity: a retrospective evaluation of tolerance and efficacy. Obes Surg. 2004;14(4):539–44.
Doldi SB, Micheletto G, Di Prisco F, et al. IGB in obese patients. Obes Surg. 2000;10(6):578–81.
Al-Momen A, El-Mogy IIGB. For obesity; a retrospective evaluation of tolerance and efficacy. Obes Surg. 2005;15(1):101–5.
Herve J, Wahlen CH, Schaeken A, et al. What becomes of patients 1 year after the IGB has been removed? Obes Surg. 2005;15(6):864–70.
Melissas J, Mouzas J, Filis D, et al. The IGB—smoothing the path to bariatric surgery. Obes Surg. 2006;16(7):897–902.
Busetto L, Segato G, De Luca M, et al. Preoperative wt loss by IGB in super obese patients treated with laparoscopic gastric banding: a case control study. Obes Surg. 2004;14(5):671–6.
Doldi SB, Micheletto G, Perrini MN, et al. Treatment of morbid obesity with IGB in association with diet. Obes Surg. 2002;12(4):583–7.
Conference NIH. Gastrointestinal surgery for severe obesity. Consensus development conference panel. Ann Intern Med. 1991;115:956–61.
Machytka E, Marinos G, Kerdahi R, Srivastava ED, AlLehibi A , Mason J, Brooks J. Spatz adjustable balloons: results of adjustment for intolerance and for weight loss plateau. DDW 2015, IFSO 201
Brooks J, Tsvang E, Ganon M. Arnon R. In: Spatz3 adjustable balloon: early adjustment to prevent premature extraction.DDW; 2016.
Gomez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity. 2016;24:1849–53.
Machytka Evzen, Puig Divi Valenti, Saenger Fernando, Sorio Ricardo, Brooks Jeffrey. Adjustable Balloons for Weight Loss: A higher yield of responders compared with non-adjustable balloons. DDW 2017 abstract. Submitted for publication Jan 2017.
Mathus-Vliegen EM, Tytgat GNJ. Intragastric balloon treatment for obesity: safety, tolerance and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61(1):19–27.
Negrin Dastis S, François E, et al. Intragastric balloon for wt loss: results in 100 individuals followed for at least 2.5 years. Endoscopy. 2009;41:575–80.
Kahtani KA, Khan MQ, Helmy A, et al. Bio-enteric intragastric balloon in obese patients: a retrospective analysis of King Faisal Specialist Hospital experience. Obes Surg. https://doi.org/10.1007/s11695-008-9654-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Conflict of Interest
Dr. Eduardo Usuy Jr. has no conflict of interest to report. Dr. Jeffrey Brooks is a shareholder in Spatz FGIA, Inc.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Usuy, E., Brooks, J. Response Rates with the Spatz3 Adjustable Balloon. OBES SURG 28, 1271–1276 (2018). https://doi.org/10.1007/s11695-017-2994-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2994-x