Abstract
Summary
Still little is known about the manifestations of osteogenesis imperfecta (OI) in adults. We therefore initiated this study of bone mass, bone turnover and prevalence of fractures in a large cohort of adult patients. We found a surprising low prevalence (10%) of osteoporosis. These patients, however, expressed the most severe disease.
Purpose
To characterize bone mineral density, bone turnover, calcium metabolism and prevalence of fractures in a large cohort of adults with osteogenesis imperfecta.
Methods
One hundred fifty-four patients with adult OI participated and 90 (age range 25–83) provided dual X-ray absorptiometry (DXA) measurements. According to Sillence classification criteria, 68 persons were classified as OI type I, 9 as type III, 11 type IV and 2 were unclassified. Fracture numbers were based on self-reporting. Biochemical markers of bone turnover were measured and bone mineral density (BMD) of the spine, femoral neck and total body were determined by DXA.
Results
Only 10% of adults with OI exhibited osteoporotic T scores (T ≤ −2.5) but compared to patients with normal T scores this subgroup had a threefold higher fracture risk (22 vs. 69). s-PTH, s-Ca and 25[OH] vitamin D were all normal. Bone markers did not display major deviations from normal, but patients with OI type III displayed higher resorption marker levels than type I and IV. Multivariate regression analysis showed that only gender and total body BMD were significant determinants of fracture susceptibility, and the differences for total body BMC, BMD and Z scores were significant between the OI subtypes.
Conclusions
In adult OI, DXA measurements only identified few patients as osteoporotic. These patients, however, exhibited a much higher fracture propensity. Due to deformities, low body height and pre-existing fractures, DXA assessment is complicated in this disease, and further studies are needed to work out how to minimize the impact of these confounders.
Similar content being viewed by others
Introduction
Osteogenesis imperfecta (OI) is the most common of the genetic connective tissue disorders that primarily affect bone. In the majority of patients with OI, the disease has been linked to mutations in one of two genes coding for collagen type I α chains (COL1A1 and COL1A2) [1], but in some patients with the typical phenotypic characteristics of OI, no such mutations were found. The most commonly used classification system is based on the Sillence criteria and distinguishes four clinical subtypes of OI [2]. Over the last years, however, several new subtypes have been added to this classification [3–5].
Fractures after minimal trauma constitute the main clinical feature of OI. In the past, OI was often regarded as a form of osteoporosis. However, contrary to postmenopausal osteoporosis, where loss of bone mass and structure predominate, OI is characterized by increased bone fragility due to defective matrix quality caused by defective type I collagen [6, 7]. The fracture rate in OI is high in the prepubertal years, declines in the post-pubertal years and rises again after menopause in women and between 60 and 80 years of age in men [8–10]. In OI the regulation of calcium metabolism is generally unperturbed with serum levels of Ca, parathyroid hormone (PTH) and vitamin D metabolites within the reference range in most patients [10–12]. Some centres have however reported low vitamin D levels in a significant proportion of patients [13].
While assessment of bone mineral density (BMD) by dual X-ray absorptiometry (DXA) and bone turnover markers have been used extensively in children, in particular for the evaluation of medical treatment with bisphosphonates [14–16], few data pertaining to these variables have been reported in adults with OI [17, 18]. However, questions about osteoporosis are often brought up by the patients with OI when they reach their adult life, and many of them worry about the clinical consequences of a combination of OI and age-related bone loss. In subjects with OI, no consensus on bone turnover levels has been reached. Bone turnover has been described as both normal to low [19–21] or increased [18, 22–24].
In order to better describe the prevalence of fractures, bone mineral density, bone turnover and calcium metabolism in adults with OI, we initiated this study in a large cohort of Norwegian patients with adult OI. The diagnosis of OI was based on clinical characteristics according to Sillence classification and family history. By measuring BMD we also wanted to study how common osteoporosis, as defined by WHO criteria [25], is in adult patients with OI, and whether BMD was related to Sillence type, and prevalence of fractures.
Methods
Material
The present study was part of a larger study describing an adult population with OI in Norway, aged 25 years and older [26]. This age limit was chosen as it is anticipated that the peak bone mass has been obtained at 25 years of age in persons with a normal skeleton [27]. Adults with OI registered at The National Resource Centre for Rare Disorders (TRS) were invited to participate in the study (n = 154) of which 97 agreed to participate. The prevalence of clinically diagnosed OI in a Caucasian population has been reported to be about 5 of 100,000 [28], and in Norway, this should correspond to a total number of persons with OI in the range of 200–300. On a voluntary basis, TRS has registered 259 persons with OI, but there are however no studies of the completeness of this registry. The type of OI was classified using the Sillence criteria [2], and 95 patients who fulfilled the criteria of OI type I (75 persons), type III (9 persons) or type IV (11 persons) were included [26]. Two persons were unclassified and are not included in all analyses. The study was approved by the regional medical research ethics committee. Each of the participants gave their written consent.
Data collection
Fracture history
All participants underwent a structured interview concerning medical history and a clinical examination. The total number of fractures was self-reported by the patients during the interview. No further ascertainment of fractures was done. Uncertainties related to recall of the total number of fractures were also registered. This uncertainty was divided into four categories: (1) certain—the exact number of fractures, 2) slightly uncertain—variation of five fractures, (3) uncertain—variation of more than five fractures and (4) very uncertain.
Bone mineral density
BMD (g/cm²) of the lumbar vertebrae L2–L4 in the anterior–posterior projection, total hip and the total body skeleton were determined by DXA (Lunar DPX-1; Lunar, WI, USA). As bone mass is sex and age specific, the individual BMD values were converted into Z and T scores based on reference values of the manufacturer. Earlier analyses demonstrated that this normal range was comparable to values derived from a Norwegian cohort [29]. The coefficients of variation (CV%) for BMD in persons without OI in our laboratory were 1.1% for the lumbar spine, 1.0% for the femoral neck, 1.4% for the total hip and 0.9% for the total body. The CV% for bone mineral content (BMC) was 2.0% for the lumbar spine and 0.8% for the total body.
Assessment of calcium metabolism and biochemical markers of bone turnover
Blood tests were drawn and second void morning urine were sampled in the fasting state between 8 and 9 o'clock in the morning and analyzed as routine tests at the Hormone Laboratory, Aker University Hospital. Twenty-five percent of the participants were using supplement of calcium and vitamin D (Table 1).
Bone turnover markers
Serum levels of osteocalcin were measured by luminoimmunoassay (BRAHMS Diagnostica GMBH, Berlin, Germany), C-terminal telopeptide of type I collagen generated by metalloproteinases (1CTP) by radioimmunoassay (Orion Diagnostica, Espoo, Finland) and bone-specific alkaline phosphatase (bALP) by enzyme activity measurement (Metra Biosystems Inc., CA, USA). The urine concentration of N- telopeptides type I collagen (NTX) were measured by enzyme immunoassay (Ostex, Seattle, WA, USA) and corrected for urine creatinine concentration.
Calcium metabolism
Serum levels of 25-hydroxyvitamin D (25(OH)D) were measured by radioimmunoassay (DiaSorin, Stillwater, MN, USA), and serum levels of intact PTH by chemiluminoimmunometric assay (DPC, Los Angeles, CA, USA). Ionized calcium levels were measured using the 634 Ca²+/pH analyzer from Ciba Corning.
Statistical methods
Descriptive statistics were reported for the different types of OI according to Sillence.
Due to the strongly skewed distributions of the total number of fractures, linear regression analysis was performed after the log transformation. Linear regression analysis was also performed to look at possible associations between the total number of fractures, OI type, and age (divided into the following groups: all women, women <50 years, women ≥50 years, all men). Comparisons of the total number of fractures in women ≥50 years and <50 years were performed by one-way ANOVA. Comparisons of the bone markers data across groups were done by one-way ANOVA, and corresponding post hoc tests with Bonferroni correction. P values ≤0.05 were considered statistically significant. All analyses were carried out using the Statistical Package for the Social Sciences, SPSS, version 15.0.
Results
Demographic and ambulatory characteristics of the study population are listed in Table 1. Seventeen persons (16 females and 1 male) were using bisphosphonates and/or hormone replacement therapy. There were no significant differences in anti-osteoporosis treatment between OI subtypes. Twelve persons had suffered new fractures or undergone orthopaedic procedures during the last year. Routine biochemistry exhibited few abnormalities: One patient with revealed increased serum glucose (15.9 mmol/l); one suppression of TSH (FT4 = 18.50 pmol/l) and two males exhibited increased TSH (FT4 = 14.5 and 14.9 pmol/l). All patients showed normal kidney function with serum creatinine below 110 umol/l, except for one elderly patient (83 years) with a value of 140 umol/l.
Prevalence and localisation of fractures
Table 2 shows the median numbers of total fractures for the study population and the median numbers of total fractures related to gender and OI type. The degree of uncertainty expressed by the patients regarding the total number of fractures sustained in any given individual showed that about 86% of the participants were quite certain about the number of fractures they had sustained (variation of five fractures or less). However, those who had sustained a high number of fractures expressed a higher degree of uncertainty (>±5 fractures).
Nearly all the participants had fractures affecting the long bones of the arms and legs or small bones of hands and feet. Thirty-two percent reported vertebral fractures, however 51% of the participants had never been tested and did not know whether they had suffered vertebral fractures or not. Fifty-four percent reported fractures at other locations as ribs, clavicle, mandible/maxilla, nose, pelvis and scapula. There were no significant differences in number of prevalent fractures between those who used bisphosphonates and those who did not.
Determinants of fracture susceptibility
Univariate regression analyses revealed significant associations between OI type, sex, total body BMC and BMD (total body and total hip) and number of fractures. After multiple regression analyses did gender (males) and total body BMD remained significant predictors for the number of fractures, while the effects of the other predictors became nonsignificant.
No significant correlation between the total number of fractures and women's age (p = 0.133), women <50 years of age (p = 0.968), women ≥50 years (p = 0.061), men's age (p = 0.226) or the age in OI type I (p = 0.110) was demonstrable. These analyses were not performed in groups with OI type III and IV due to the low number of patients in these groups.
Bone mineral density
Seven participants were not measured by DXA (2 were pregnant, 1 could not be examined in the supine position, 4 did not want to participate in this part of the study), leaving 90 for DXA assessment. However, DXA of the spine and hip were confounded by considerable problems in technical analyses due to pathology such as metal implants and deformities. For this reason, 48 DXA measurements of the spine and 22 DXA measurements of the total hip were not included in the results. During assessment of lumbar spine BMD values, vertebrae with obvious compression fracture were excluded. As the software program excludes areas with metal implants, patients with such implants were not excluded from the total body measurements.
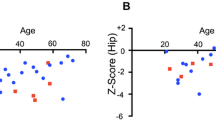
Summary data of bone mass (BMC) and BMD for the study population, and for the different OI types, are presented in Table 3. We found significant differences between Sillence type III and type I and IV with respect to total body BMC (p < 0.001), total body BMD (p < 0.001) and total body BMD Z score (p = 0.02). Skeletal pathologies in patients with OI type III prevented us from getting any measurements of the spine and the hip in this group. Type IV revealed a significantly lower L2–L4 Z score (p = 0.01) than type I, but no difference in total hip Z score was found.
Osteopenia and osteoporosis
Total body BMD T scores showed a wide variation, ranging between −4.70 and +1.90. For the different subtypes, the range did not differ significantly. Mean T scores for the different subtypes ranged between −0.61 for type I and −2.74 for type III in the total body. No significant differences between types of OI were found at the hip (no OI type III included) (Table 4). We found significant differences for the total body BMD T score, between OI type I and type III and between type IV and type III (p < 0.001), with type III exhibiting the lowest values. No significant differences were found between type I and type IV (p = 1.0).
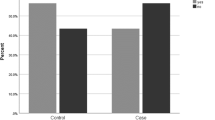
Using the WHO criteria for osteopenia (T score between −1.0 and −2.5) and osteoporosis (T score ≤−2.5) [25], 27 (30%) of the participants fulfilled the criteria for osteopenia and 9 (10%) for osteoporosis on total body BMD (Table 4). Based on total hip BMD T scores, 34 (50%) of the participants fulfilled the criteria for osteopenia and 10 (21%) for osteoporosis.
Calcium metabolism
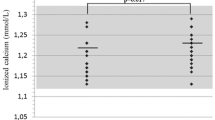
s-PTH, s-Ca and 25(OH) vitamin D levels were normal in all OI subtypes (Table 5). Sixteen participants exhibited 25(OH) vitamin D values <50 nmol/l, but only one revealed signs of secondary hyperparathyroidism (s-PTH 8.5 pmol/l). Persons with OI type III displayed significantly lower values for 25 vitamin (OH) D (p = 0.05) than persons with OI type I and IV.
Bone turnover
Patients using bisphosphonates and/or hormone replacement therapy (HRT, n = 17) were excluded from the following calculations. Patients who had sustained fractures and/or had orthopaedic operations the last 12 months (n = 12) did not display significant deviations pertaining to bone markers and bone mineral density measurements and were therefore not excluded from these analyses. Figure 1 shows serum levels for two markers of bone formation (osteocalcin and bALP) and three markers of bone resorption [1CTP, deoxypyridinoline crosslinks (DPYD) and NTX]. Generally, patients with OI type III displayed slightly lower formation maker levels.
1CTP was significantly higher in OI type III than in OI type I (p = 0.02) and DPYD was significantly higher in OI type III than in OI type IV (p = 0.05). NTX did not show significant differences across the groups.
Discussion
The aim of this study was to ascertain number of fractures, bone density, bone turnover and calcium metabolism in an adult population with OI. Only 17 persons in our study population were using bisphosphonates and/or HRT (13 were using bisphosphonates), which gave us the opportunity to investigate bone mineral density and metabolism in adults with OI unaffected by pharmaceuticals affecting bone metabolism. Increased knowledge in this field is needed to create adequate guidelines for treatment and follow-up of OI in adult life.
Several papers have reported that fractures in women with OI tend to occur mostly before puberty, decrease through adulthood and then increase after menopause [8, 30]. Paterson et al. found that the fracture rate rose after menopause in women and remained low after adolescence in men. This increase in fractures in postmenopausal women was considered a result of OI combined with age-related bone loss. In our study we were unable to corroborate this finding. We found no correlation between age and the total number of fractures, neither in women nor in men. Although we have not had the possibility to measure the age-specific incidence rate of clinical fractures, we think that the reported prevalence data indicate that there is no increase in rate in the elderly women and men. There were no significant differences in the total number of fractures between women before and after 50 years of age, as would have been expected if the age-related bone loss had been superimposed on the effect of OI. Our hypothesis explaining this phenomenon is that the poor matrix quality of OI bone due to the collagen defects overrides other age- and menopause-dependent changes in bone mass and structure. Concerning OI type and total number of fractures, our study showed no correlation between total number of fractures and age in OI type I. The numbers of OI type III and IV were too small to make this analysis.
With respect to the localization of fractures, our findings are in accordance with the literature [9]. Long bones of the arms and legs, and small bones of the hands and feet were most frequently broken. As more than half of the patients never had a spinal X-ray examination, the estimation of spine fracture prevalence in this cohort was impossible.
Bone mineral density and osteoporosis
Few studies have examined the role of BMD measurements in the diagnosis or as a risk factor for fracture in adult OI. Although BMD is not essential for establishing the diagnosis of OI, it appears to be an indicator of disease severity and may be predictive of long-term functional outcome [31]. As in osteoporosis, the low BMD is probably a major risk factor for further fractures. However, few adult patients with OI have been investigated using BMD. In our study more than 50% of the participants had previously never had a BMD measurement, and none of them had been subjected to regular DXA follow-up.
Several studies have measured BMD in children [14–16] and in adults [10, 17] with OI. Most of them have measured the lumbar spine and/or the hip. In our cohort, especially for OI types III and IV, measurements from the lumbar spine and the hip could not be analyzed due to considerable deformities. Adults with OI often have scoliosis which makes any form of spine measurement difficult [32]. Another important issue is the lack of overall agreement on how to adjust BMC for body size, thereby creating BMD values, in adults with OI. In multivariate analysis, gender and total body BMD were significant determinants of fracture susceptibility, no significant impact of OI type, total hip BMD and total body BMC was demonstrable. As total body BMD is technically easier to perform in OI patients than spine and hip, total body BMD probably should be used as the preferred BMD estimate in patients with OI.
Persons with OI are often described as osteoporotic, but BMD values in OI have also been reported as normal [32]. In the absence of consensus on how to characterize possible osteoporosis in adults with OI, we chose to use established criteria for osteoporosis. Using the WHO criteria on total body BMD, about 30% of the total OI population had osteopenia, and 10% were osteoporotic. Remarkably, only 4% with OI type I were osteoporotic (T score <−2.5) based on total body BMD, while 25% were classified as osteoporotic using total hip BMD.
Serum calcium has been reported as generally normal in patients with OI [11, 12]. Serum 25-hydroxyvitamin D may be low [13]. In our study we found normal S-PTH, s-Ca and vitamin D levels in serum. Subjects with OI type III, all wheelchair users, however, displayed significantly lower values for 25(OH)D than persons with OI type I and IV. Low values for vitamin D will tend to increase the bone turnover due to secondary hyperparathyreoidism, and we found that all resorption markers were increased in OI type III compared to OI types I and IV, although PTH was not increased in OI type III. However, these results should be evaluated with caution, as we did not include a control group, but only relied on the reference ranges of the laboratory. Previous reports give no clear direction in terms of bone turnover markers. Reduced, normal and increased levels have been reported [18–24].
There are some limitations in this study. The study population included about two thirds of the registered Norwegian adult OI population, but as OI is a rare disease, the sample size was still small, especially in the groups of OI type III and IV. This makes it difficult to compare the groups. We do not have the possibility to perform collagen analysis in our country, and the diagnosis of OI was therefore based on clinical examination and family history. Because the total number of fractures was based on information from the patients, and not possible to verify, this information was uncertain. However, the degree of uncertainty was clearly associated with the total number of fractures. Those who expressed the highest uncertainty had the highest number of fractures.
Conclusions
In conclusion, total body BMD is probably the best estimate in adults with OI. However, due to deformities, low body height and pre-existing fractures, DXA assessment is complicated in this disease, and further studies are needed to work out how to minimize the impact of these confounders. Our analyses of bone mineral density indicate that only 10% of patients displayed osteoporotic T scores, further supporting the notion that bone fragility in OI mainly stems from defective collagen structure. These patients, however, exhibited a much higher fracture propensity than patients with normal T scores. The levels of bone turnover markers were normal in the vast majority of patients. However, in adults with OI type III, bone turnover tended to be increased and osteoporosis more prevalent.
References
Rowe WD, Shapiro RY (1990) Osteogenesis imperfecta. In: Avioli L, Krane S (eds) Metabolic bone disease and clinical related disorders, 2nd edn. WB Saunders, USA, Philadelphia, pp 659–701
Sillence DO, Senn A, Danks DM (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 15:101–116
Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P et al (2009) Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res 15:1650–1658
Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R (2002) Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res 17:30–38
Ward LM, Rauch F, Travers R, Chabot G, Azouz EM, Lalic L, Roughley PJ, Glorieux FH (2002) Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone 31:12–18
Roughly PJ, Rauch F, Glorieux FH (2003) Osteogenesis imperfecta—clinical and molecular diversity. Eur Cell Mater 5:41–47
Viguet-Carrin S, Garnero P, Demas PD (2006) The role of collagen in bone strength. Osteoporos Int 17:319–36
Paterson C, McAllon S, Stellman JL (1984) Osteogenesis imperfecta after the menopause. N Eng J M 310:1694–1696
Primorac D, Rowe DW, Mottes M, Barišić I, Antičević D et al (2001) Osteogenesis imperfecta at the beginning of bone and joint decade. CMJ 42:393–415
Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, Tatơ L (2003) Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res 18:126–130
Whyte MP (1999) Osteogenesis imperfecta. In: Favus MJ (ed) Primer on the metabolic bone diseases and disorders of mineral metabolism, 4th edn. Lippincott-Williams and Wilkins, Philadelphia, pp 386–389
Rauch F, Plotkin H, Travers R, Zeitlin L, Glorieux FH (2003) Osteogenesis imperfecta types I, III and IV: effect of pamidronate therapy on bone and mineral metabolism. J Clin Endocrinol Metab 88:986–992
Meunier PJ (1999) Serum 25-hydroxy-vitamin D is often low, indicating vitamin D deficiency secondary to lack of exposure to sunlight, Abstractbook, 7th International Conference on Osteogenesis Imperfecta, Montreal, 1999
Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers C (1998) Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Eng J Med 339:947–952
Åstrøm E, Söderhall S (2002) Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child 86:356–364
Rauch F, Travers R, Plotkin H, Glorieux FH (2002) The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest 110:1293–1299
Gatti D, Colapietro F, Fracassi E, Sartori E, Antoniazzi F, Braga V, Rossini M, Adami S (2003) The volumetric bone density and cortical thickness in adult patients affected by osteogenesis imperfecta. J Clin Densitom 6:173–177
Braga V, Gatti D, Rossini M, Colapietro F, Battaglia E, Viapiana O, Adami S (2004) Bone turnover markers in patients with osteogenesis imperfecta. Bone 34:1013–1016
Cepollaro C, Gonelli S, Pondrelli C, Montagnani A, Martini S, Bruni D, Gennari C (1999) Osteogenesis imperfecta: bone turnover, bone density and ultrasound parameters. Cacif Tissue Int 65:129–132
Lund AM, Hansen M, Kollerup G, Juul A, Teisner B, Skovby F (1998) Collagen-derived markers of bone metabolism in osteogenesis imperfecta. Acta Paediatr 87:1131–1137
Shapiro JR (1995) Comments. J Bone Miner Res 10:338–339
Brenner RE, Vetter U, Bollen AM, Morike M, Eyre DR (1994) Bone resorption assessed by immunoassay of urinary cross-linked collagen peptides in patients with osteogenesis imperfecta. J Bone Miner Res 9:993–997
Rauch F, Travers R, Parfitt AM, Glorieux FH (2000) Static and dynamic bone histometry in children with osteogenesis imperfecta. Bone 26:581–589
Chines A, Petersen DJ, Schrank FW, Whyte MP (1991) Hypercalciuria in children severely affected with osteogenesis imperfecta. J Pediatr 119:51–57
WHO Scientific Group on the Prevention and Management of Osteoporosis (2000: Geneva, Switzerland) Prevention and management of osteoporosis: report of a WHO scientific group. (WHO Technical Report Series No 921)
Wekre LL, Froslie KF, Haugen L, Falch JA (2010) A population-based study of demographical variables and ability to perform activities of daily living in adults with osteogenesis imperfecta. Disabil Rehabil 32:579–587
Teegarden D, Proulx WR, Martin BR, Zhao J, McCabe GP, Lyle RM, Peacock M, Slemenda C, Johnston CC, Weaver CM (1995) Peak bone mass in young women. J Bone Miner Res 10:711–715
Marini JC (1988) Osteogenesis imperfecta: comprehensive management. Adv Pediatr 35:391–426
Gjesdal CG, Aanderud SJ, Haga HJ, Brun JG, Tell GS (2004) Femoral and whole-body bone mineral density in middle-aged and older Norwegian men and women: suitability of the reference values. Osteoporos Int 15:525–534
Chevrel G Osteogenesis imperfecta. Orphanet encyclopedia, June 2004. Available at: http://www.orpha.net/data/patho/GB/uk-OI.pdf
Huang RP, Ambrose CG, Sullivan E, Haynes RJ (2006) Functional significance of bone density measurements in children with osteogenesis imperfecta. JBJS Am 88:1324–1330
Paterson CR, Mole PA (1994) Bone density in osteogenesis imperfecta may well be normal. Postgrad Med J 70:104–107
Acknowledgments
The authors thank the Norwegian Foundation for Osteogenesis Imperfecta, all those who participated in the study for their time and efforts, statistician Kathrine Frey Frøslie for statistical advice and all our co-operators at Aker University Hospital and TRS National Resource Centre who made the different investigations possible.
Conflicts of interest
No disclosures.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support
The Norwegian Foundation for Health and Rehabilitation
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Wekre, L.L., Eriksen, E.F. & Falch, J.A. Bone mass, bone markers and prevalence of fractures in adults with osteogenesis imperfecta. Arch Osteoporos 6, 31–38 (2011). https://doi.org/10.1007/s11657-011-0054-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11657-011-0054-z