Abstract
Background
Older adults face high mortality following resuscitation efforts for in-hospital cardiac arrest. Less is known about the role of frailty in survival to discharge after in-hospital cardiopulmonary resuscitation.
Objective
To investigate whether frailty, measured by the Clinical Frailty Scale, is associated with mortality after cardiopulmonary resuscitation following in-hospital cardiac arrest in older adults in the USA.
Design
Retrospective cohort study.
Participants
Patients ≥ 65 years who had undergone cardiopulmonary resuscitation during an inpatient admission at two urban academic hospitals and three suburban community hospitals within a Boston area healthcare system from January 2018-January 2020. Patients with Clinical Frailty Scale scores 1–3 were considered not frail, 4–6 were considered very mildly, mildly, and moderately frail, respectively, and 7–9 were considered severely frail.
Main Measures
In-hospital mortality after cardiopulmonary resuscitation.
Key Results
Among 324 patients who underwent cardiopulmonary resuscitation following in-hospital cardiac arrest, 73.1% experienced in-hospital mortality. Patients with a Clinical Frailty Scale score of 1–3 had 54% in-hospital mortality, which increased to 66%, 78%, 84%, and 84% for those with a Clinical Frailty Scale score of 4, 5, 6, and 7–9, respectively (p = 0.001). After adjusting for age, sex, race, and Charlson Comorbidity Index, higher frailty scores were significantly associated with higher odds of in-hospital mortality. Compared to those with a Clinical Frailty Scale score of 1–3, odds ratios (95% CI) for in-hospital mortality for patients with a Clinical Frailty Scale score of 4, 5, 6, and 7–9 were 1.6 (0.8–3.3), 3.0 (1.3–7.1), 4.4 (1.9–9.9), and 4.6 (1.8–11.8), respectively (p = 0.001).
Conclusions
Higher levels of frailty are associated with increased mortality after in-hospital cardiopulmonary resuscitation in older adults. Clinicians may consider using the Clinical Frailty Scale to help guide goals of care conversations, including discussion of code status, in this patient population.
Similar content being viewed by others
INTRODUCTION
Mortality following cardiopulmonary resuscitation (CPR) in hospitalized adults approaches 80%.1 For many older adults and their loved ones, choosing to undergo or forgo CPR is a complex and emotionally challenging decision, fraught with uncertainty. Knowing the likely outcome of an intervention is critical to productive goals of care conversations.2,3 Many people make decisions based on their understanding of prognosis, with a majority of individuals choosing to allow natural death (e.g., a do not resuscitate/do not intubate (DNR/DNI) code status) after learning the reported likelihood of survival after CPR.4 However, tools currently available to clinicians are limited in predicting survival. Although age is independently associated with poor outcomes after CPR.5, the older adult population is heterogeneous, and age alone is an unreliable predictor of adverse outcomes.6 Clinicians, patients, and families need high-quality tools to identify in whom CPR is likely to preserve life and in whom it is not.
Frailty, defined as a reduction in physiologic reserve that results in a decreased ability to tolerate stressors,7,8,9 is increasingly recognized as a framework for identifying older adults at risk for adverse health outcomes. Although no universally accepted definition of frailty exists, a wide variety of clinical and research tools are available to identify and grade frailty.10,11 The Clinical Frailty Scale (CFS), a nine-point visual and descriptive scale, was designed to be rapidly applied in clinical practice.12,13,14 Previous investigations demonstrated that increased frailty is an independent risk factor for mortality after in-hospital cardiac arrest.15,16,17 To our knowledge, studies have not examined the association between frailty and mortality in older adults following in-hospital CPR in the USA. In this study, we sought to investigate whether frailty is associated with mortality after CPR following in-hospital cardiac arrest in older adults. We hypothesized that older adults with a greater level of frailty would have a higher risk of mortality following in-hospital CPR.
METHODS
Study Design and Population
This was a retrospective cohort study conducted within the Mass General Brigham Integrated Health Care System (MGB). The MGB inpatient volume captures approximately 18% of Massachusetts discharges per year. The included hospitals within MGB comprised of two academic (Massachusetts General Hospital and Brigham & Women’s Hospital) and three community hospitals (Brigham and Women’s Faulkner Hospital, Newton Wellesley Hospital, and North Shore Medical Center). For this study, we included all patients aged 65 years and older who underwent CPR following an in-hospital cardiac arrest between January 1, 2018, and January 31, 2020. Patients were identified using a centralized clinical data registry for MGB, the Research Patient Data Registry (RPDR).18 Patients who underwent CPR in the field or in the emergency department were excluded. Our study was conducted in accordance with STROBE guidelines for observational studies.19
CFS Score Extraction
Our research team employed an iterative process to develop a standardized protocol for calculating a CFS score based on retrospective chart review. First, two geriatricians who use the CFS in clinical practice (ARO and SSt) developed an algorithm for generating a CFS score from chart review, including a structured workflow for extracting information from the electronic medical record regarding functional status, comorbidity and symptom burden, and exercise tolerance that is necessary for calculating a CFS (Fig. 3 in the Appendix). The algorithm was modified from the original classification tree in accordance with the data available in our electronic medical record. We additionally solicited feedback on the algorithm from a third author (OT), who participated on the research team that first developed and validated the CFS classification tree score.20,21 CFS scores were generated by applying the algorithm, in conjunction with clinical judgment based on chart review. Patients found to have CFS scores 1–3 were classified as not frail. Those with CFS scores 4–6 were classified as very mildly, mildly, and moderately frail, respectively, and those with CFS scores 7–9 as severely frail.22
Validity and Reliability of the CFS Scoring
To assess validity of the CFS scoring using the algorithm as an aid, we applied it to ten charts of patients seen in the prior 3 months by a member of the Brigham and Women’s Hospital inpatient geriatrics consult service and for whom a Geriatrician documented both a comprehensive geriatric assessment23 and an in-person CFS score. Two members of the research team (ARO and SSt) calculated CFS scores without reference to the geriatrics consult documentation and subsequently compared the CFS scores obtained using the algorithm as an aid to the scores obtained by the in-person comprehensive geriatric assessment. Seven out of 10 CFS scores matched exactly, and the remaining three scores were within one point of each other.
To assess reliability of our retrospective CFS scoring process, we then applied it to groups of five charts from our study cohort. Two members of the research team independently scored a CFS for each chart and then compared results; in two rounds of five charts, eight out of 10 scores matched exactly, and the remaining two scores were within one point of each other. We then divided 370 charts evenly between three of the authors (FYH., LO, and SMS). To confirm that inter-rater reliability remained high, 10% of the charts were randomly selected to be scored a second time by a third reviewer who was blinded to the others’ scores (SSt). Inter-rater reliability was > 90%. Score discrepancies greater than one point were discussed by the whole team until we achieved consensus. Because frailty is dynamic and expected to change over time,24 46 patients with insufficient information in the chart to calculate a CFS score in the three months prior to cardiac arrest were excluded.
Outcome
The primary outcome was survival to hospital discharge, as reported in the RPDR.25 Secondarily, we examined 30-day mortality, a categorical variable determined by calculating whether number of days between CPR date and date of death was > 30. CPR date and date of death were also extracted from the RPDR.
Other Covariates
Patient data were extracted from the RPDR. Demographic variables included age, sex, race, ethnicity, and primary language. Comorbidity variables were determined using ICD-10 diagnosis codes for hypertension, hyperlipidemia, coronary artery disease, myocardial infarction, congestive heart failure, atrial fibrillation, peripheral vascular disease, cerebrovascular disease, hemiplegia, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, chronic kidney disease, history of heart/lung/kidney transplant, diabetes mellitus, malignancy, and HIV/AIDS. Patient age and comorbidities (myocardial infarction, congestive heart failure, atrial fibrillation, peripheral vascular disease, cerebrovascular disease, hemiplegia, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease (mild or moderate/severe), moderate/severe chronic kidney disease, diabetes mellitus (with or without complications), malignancy (presence of or metastatic carcinoma), and AIDS) were used to calculate a Charlson Comorbidity Index.26
Statistical Analysis
Prior to data collection, a sample size calculation was conducted to determine the study sample size needed to determine a significant association between frailty, as determined by the CFS, and the primary outcome, in-hospital mortality. Based on previous literature,27 we anticipated that in-hospital mortality in this cohort of hospitalized older adults would be 85–90%. To detect a 25% difference in in-hospital mortality between frail and non-frail groups, with confidence interval of 95%, alpha of 0.05 and power of 80%, we aimed to collect data for at least 51 subjects per group.
We performed descriptive analyses for patient demographics, comorbidities, and outcomes and determined mortality rates for each CFS score. We performed bivariate analyses using chi-square tests to measure the association between patient demographics (age ≥ 75 years, sex), comorbidities (Charlson Comorbidity Index ≥ 9.8, history of heart/lung/kidney transplant, and history of coronary artery disease/congestive heart failure/arrhythmia), CFS score, and the outcome of in-hospital mortality. Logistic regression was performed to measure the association between frailty status and in-hospital mortality. Multivariable analysis was also used to measure the association between frailty status and in-hospital mortality, controlling for patient age alone as well as patient age, sex, race, and Charlson Comorbidity Index. Statistical analysis was performed using SAS software, version 9.4 (Cary, NC).
The Massachusetts General Brigham Institutional Review Board (IRB) approved this study.
Results
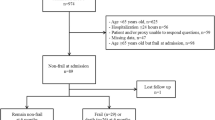
In total, there were 324 patients ≥ 65 years of age who underwent CPR following in-hospital cardiac arrest in our hospital system (Fig. 1). Mean patient age was 76.7 years (SD 7.4 years). Of these, 63.9% were male, and 79.9% were White. The most common comorbidity was hypertension, present in 78.1% of patients. With respect to cardiovascular disease, 47.5% of patients had coronary artery disease, and 68.2% had experienced a myocardial infarction. Patient demographics and co-morbidities are shown in Table 1. The average Charlson Comorbidity Index was 9.8 (SD 3.9).
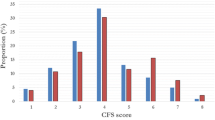
In this cohort, 17.6% (n = 57) had a CFS score of 1–3 (not frail), 26.5% (n = 86) had a CFS score of 4 (very mildly frail), 15.7% (n = 51) had a CFS score of 5 (mildly frail), 24.7% (n = 80) had a CFS score of 6 (moderately frail), and 15.4% (n = 50) had a CFS score of 7–9 (severely frail). Overall, in-hospital mortality following in-hospital CPR was 73.1% (n = 237), and 30-day mortality was 74.4% (n = 241). A higher CFS score was associated with a greater risk of in-hospital mortality following CPR (Fig. 2). Of patients with a CFS of 1–3, 54.0% (n = 31) experienced in-hospital mortality, compared to 66.3% (n = 57) of those with a CFS of 4, 78.4% (n = 40) with a CFS of 5, 83.7% (n = 67) with a CFS of 6, and 84.0% (n = 42) with a CFS of 7–9 (p = 0.001). Additional bivariate analyses did not show significant associations between patient demographics or comorbidities and in-hospital mortality.
In multivariable analysis, frailty status was associated with increasing odds of in-hospital mortality (Table 2). Controlling for patient age, sex, race, and Charlson Comorbidity index, the adjusted odds ratios were 1.6 (95% CI 0.8–3.3) for a CFS of 4, 3.0 (95% CI 1.3–7.1) for a CFS of 5, 4.4 (95% CI 1.9–9.9) for a CFS of 6, and 4.6 (95% CI 1.8–11.8) for a CFS of 7–9 (p = 0.001), compared to patients with a CFS of 1–3.
Discussion
In this study, we found that increasing levels of frailty, measured according to the Clinical Frailty Scale, were associated with an increased risk of in-hospital mortality following in-hospital CPR in adults aged 65 and older. Results remained the same after accounting for demographics and comorbid conditions. This work highlights the variability in prognosis following CPR among older adults and should prompt clinicians to consider frailty when engaging in shared decision-making conversations. The CFS is a simple yet highly prognostic tool that can be used by clinicians in various settings to quickly assess frailty.
In prior studies describing outcomes following in-hospital CPR in older adults, survival to hospital discharge has ranged from 9 to 32%.15,28,29,30,31 The strongest predictors of increased mortality were increasing age.28,29,30 and unshockable rhythm at the time of the arrest.28,29 These factors32,33 have also been shown to predict 1-year mortality in survivors of in-hospital cardiac arrest. While overall survival to hospital discharge in our study was 26%, survival varied significantly according to frailty status. In fact, for those who were not frail, survival approached 50%. Our findings of an increased risk of mortality with higher frailty score are similar to those reported in studies conducted in the UK, Canada, and China.15,16,17,34 However, we observed higher survival rates in patients with frailty compared to other studies. In a retrospective study from Ibitoye et al. that included 90 patients ≥ 60 years, in-hospital mortality following in-hospital CPR was 100% for patients categorized as frail, defined as CFS ≥ 5,16,17,34 while Wharton et al. examined in-hospital cardiac arrest in 179 adults at a single institution and observed 98% in-hospital mortality for patients with frailty, defined as a CFS score of ≥ 6.15 Similarly, Fernando et al. examined in-hospital cardiac arrest in 477 adults and observed 95% in-hospital mortality for frail patients, defined as a CFS score ≥ 5, while patients who were not frail had an in-hospital mortality of 67%. Meanwhile, we found that 84% of patients with a CFS ≥ 6 experienced in-hospital mortality following in-hospital CPR.
It is possible that the higher survival rates in our cohort are due in part to the robust palliative care presence within our institution which has resulted in increased attention to goals of care conversations across Mass General Brigham.35,36 Accordingly, patients that clinicians deemed unlikely to survive CPR often chose a do not resuscitate/do not intubate (DNR/DNI) code status and therefore would have been excluded from our study. Conversely, the comparatively high rates of survival in our cohort should caution clinicians against undertreatment of older adults with minimal frailty burden, who may be more likely to survive CPR than age alone would suggest. These findings emphasize the value of a readily available metric for risk stratification for in-hospital CPR in older adults. Determining a patient’s degree of frailty may help clinicians avoid both overtreatment and undertreatment, as the CFS helps distinguish between patients who are not frail and more likely to survive CPR and patients with increasing degrees of frailty who are less likely to survive CPR as their frailty burden increases.
We selected the CFS for frailty assessment as this scoring system is easy to use in busy clinical settings, including an intensive care unit or emergency department, and may be calculated from retrospective chart review with a high degree of validity and inter-rater reliability.13,37 Additionally, studies have shown that higher CFS scores correlate with a variety of adverse outcomes, including prolonged hospitalization, long-term care need, hospital readmission, and hospital and ICU mortality.7,12,14,38,39,40,41,42 Although other frailty indices have been developed for ease of use in clinical practice, the CFS has the advantage of conceptualizing frailty as a spectrum on which patients range from very fit to terminally frail. In contrast to prior studies which showed an association between in-hospital mortality with a binary designation of frailty,15,16,17 we report outcomes for a range of CFS scores. Using the CFS, we found that majority of the patients in our study scored 4–6 on the CFS, classified as very mildly, mildly, or moderately frail. This is consistent with other studies which have looked at the distribution of frailty in inpatient populations.34,43
By representing levels of frailty, we were able to provide a more nuanced depiction of the heterogeneous older adult population and better characterize the association between frailty and in-hospital mortality after CPR. Our results reflected the clinical reality that patients with frailty represent a heterogeneous population with different likely outcomes depending on their level of frailty. This has important implications for clinicians counseling patients about their likelihood of survival following CPR. Although we advocate strongly against an algorithmic application of our findings, such as recommending against CPR above a particular CFS threshold, we do advocate considering frailty to frame conversations when discussing goals of care. Most patients want to discuss prognosis with clinicians, and these discussions are critical to informed decision making. However, clinicians often hesitate to share prognosis because of uncertainty of estimates and potential harm to the patient.44 Using the CFS as prognostic information, shared in an individualized and compassionate manner, can help patients and families make difficult decisions about CPR. Moreover, because the CFS was designed to be simple and easy to apply at the bedside, it is an effective tool for use in busy clinical environments and crisis situations such as the recent COVID pandemic.45 Our results contribute to a growing body of evidence to inform likely outcomes to minimize patient harm and enable patient-centered care in frail older adults.
Our study has several strengths. Compared to prior studies examining the association between frailty status and in-hospital mortality following CPR, we had a relatively large sample size of older adults who experienced in-hospital cardiac arrest. Our cohort also included patients at both academic and community hospitals in the Boston metropolitan area to capture a more diverse population. In addition, we intentionally selected the CFS as a frailty assessment that would be easy to implement in clinical settings. For this study, we employed robust methods for retrospective CFS extraction with strong inter-rater reliability.
We acknowledge the limitations of this study. First, data for CFS extraction were abstracted through retrospective chart review and thus subject to error and omissions in documentation, potentially leading to inaccuracies in the calculation of CFS scores. Regarding our secondary outcome of 30-day mortality, we relied on the RPDR variable, date of death, which only captures patient deaths occurring within MGB and recorded in our electronic medical record, and may have been imprecise. Next, our cohort examined patients cared for within a single medical system in a single geographic region and may not be generalizable to a broader population. Additionally, our cohort excluded patients with a DNR/DNI code status and did not capture outcomes in older adults who had already opted out of CPR. Lastly, our analysis focused on the outcome of in-hospital mortality after in-hospital CPR. Further study is needed to evaluate whether frailty affects other outcomes after CPR, including cognitive or functional status, which are often critical to determining whether quality of life after CPR is goal-concordant.
Conclusions
Increasing level of frailty is associated with increased mortality after in-hospital CPR in older adults. Clinicians may consider using the CFS to help guide goals of care conversations, including discussion of code status, in this patient population.
References
Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-Hospital Cardiac Arrest: A Review. JAMA. Mar 26 2019;321(12):1200-1210. https://doi.org/10.1001/jama.2019.1696
Paladino J, Lakin JR, Sanders JJ. Communication Strategies for Sharing Prognostic Information With Patients: Beyond Survival Statistics. JAMA. Oct 8 2019;322(14):1345-1346. https://doi.org/10.1001/jama.2019.11533
White DB, Engelberg RA, Wenrich MD, Lo B, Curtis JR. Prognostication during physician-family discussions about limiting life support in intensive care units. Crit Care Med. Feb 2007;35(2):442-8. https://doi.org/10.1097/01.ccm.0000254723.28270.14
Murphy DJ, Burrows D, Santilli S, et al. The influence of the probability of survival on patients' preferences regarding cardiopulmonary resuscitation. N Engl J Med. Feb 24 1994;330(8):545-9. https://doi.org/10.1056/nejm199402243300807
Sandroni C, Nolan J, Cavallaro F, Antonelli M. In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med. Feb 2007;33(2):237-45. https://doi.org/10.1007/s00134-006-0326-z
van de Glind EM, van Munster BC, van de Wetering FT, van Delden JJ, Scholten RJ, Hooft L. Pre-arrest predictors of survival after resuscitation from out-of-hospital cardiac arrest in the elderly a systematic review. BMC Geriatr. Jul 3 2013;13:68. https://doi.org/10.1186/1471-2318-13-68
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. Mar 2 2013;381(9868):752-62. https://doi.org/10.1016/S0140-6736(12)62167-9
Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. Jun 2013;14(6):392-7. https://doi.org/10.1016/j.jamda.2013.03.022
Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. Oct 12 2019;394(10206):1365-1375. https://doi.org/10.1016/s0140-6736(19)31786-6
Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. Mar 4 2014;63(8):747-62. https://doi.org/10.1016/j.jacc.2013.09.070
Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71–2. https://doi.org/10.1016/j.jamda.2007.11.005
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. Aug 30 2005;173(5):489-95. https://doi.org/10.1503/cmaj.050051
Davies J, Whitlock J, Gutmanis I, Kane SL. Inter-Rater Reliability of the Retrospectively Assigned Clinical Frailty Scale Score in a Geriatric Outreach Population. Can Geriatr J. Mar 2018;21(1):1-5. https://doi.org/10.5770/cgj.21.263
Aliberti MJR, Szlejf C, Avelino-Silva VI, et al. COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J Am Geriatr Soc. Apr 5 2021; https://doi.org/10.1111/jgs.17146
Wharton C, King E, MacDuff A. Frailty is associated with adverse outcome from in-hospital cardiopulmonary resuscitation. Resuscitation. Oct 2019;143:208-211. https://doi.org/10.1016/j.resuscitation.2019.07.021
Fernando SM, McIsaac DI, Rochwerg B, et al. Frailty and associated outcomes and resource utilization following in-hospital cardiac arrest. Resuscitation. Jan 1 2020;146:138-144. https://doi.org/10.1016/j.resuscitation.2019.11.011
Ibitoye SE, Rawlinson S, Cavanagh A, Phillips V, Shipway DJH. Frailty status predicts futility of cardiopulmonary resuscitation in older adults. Age Ageing. Jan 8 2021;50(1):147-152. https://doi.org/10.1093/ageing/afaa104
Nalichowski R, Keogh D, Chueh HC, Murphy SN. Calculating the benefits of a Research Patient Data Repository. AMIA Annual Symposium proceedings AMIA Symposium. 2006;2006:1044-1044.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. Oct 20 2007;370(9596):1453-7. https://doi.org/10.1016/s0140-6736(07)61602-x
Theou O, Pérez-Zepeda MU, van der Valk AM, Searle SD, Howlett SE, Rockwood K. A classification tree to assist with routine scoring of the Clinical Frailty Scale. Age and ageing. 2021:afab006. https://doi.org/10.1093/ageing/afab006
Turcotte LA, Zalucky AA, Stall NM, et al. Baseline Frailty as a Predictor of Survival after Critical Care: a Retrospective Cohort Study of Older Adults Receiving Home Care in Ontario, Canada. Chest. 2021–06–01 2021; https://doi.org/10.1016/j.chest.2021.06.009
Shimura T, Yamamoto M, Kano S, et al. Impact of the Clinical Frailty Scale on Outcomes After Transcatheter Aortic Valve Replacement. Circulation. 2017–05–23 2017;135(21):2013–2024. https://doi.org/10.1161/circulationaha.116.025630
Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. Oct 23 1993;342(8878):1032-6. https://doi.org/10.1016/0140-6736(93)92884-v
Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. Feb 27 2006;166(4):418-23. doi:https://doi.org/10.1001/archinte.166.4.418
https://rc.partners.org/. https://rc.partners.org/. Accessed 6.18.2021,
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-83. https://doi.org/10.1016/0021-9681(87)90171-8
Van Gijn MS, Frijns D, Van De Glind EMM, C. Van Munster B, Hamaker ME. The chance of survival and the functional outcome after in-hospital cardiopulmonary resuscitation in older people: a systematic review. Age and Ageing. 2014–07–01 2014;43(4):456–463. https://doi.org/10.1093/ageing/afu035
Zoch TW, Desbiens NA, Destefano F, Stueland DT, Layde PM. Short- and Long-term Survival After Cardiopulmonary Resuscitation. Archives of Internal Medicine. 2000–07–10 2000;160(13):1969. https://doi.org/10.1001/archinte.160.13.1969
Hirlekar G, Karlsson T, Aune S, et al. Survival and neurological outcome in the elderly after in-hospital cardiac arrest. Resuscitation. 2017–09–01 2017;118:101–106. https://doi.org/10.1016/j.resuscitation.2017.07.013
Hayashi T, Matsushima M, Bito S, et al. Predictors Associated with Survival Among Elderly In-Patients Who Receive Cardiopulmonary Resuscitation in Japan: An Observational Cohort Study. Journal of General Internal Medicine. 2019–02–01 2019;34(2):206–210. https://doi.org/10.1007/s11606-018-4747-5
Fletcher JWA, Smith A, Walsh K, Riddick A. Low Rates of Survival Seen in Orthopedic Patients Receiving In-Hospital Cardiopulmonary Resuscitation. Geriatric Orthopaedic Surgery & Rehabilitation. 2019–01–01 2019;10:215145931881897. https://doi.org/10.1177/2151459318818972
Chan PS, Nallamothu BK, Krumholz HM, et al. Long-Term Outcomes in Elderly Survivors of In-Hospital Cardiac Arrest. New England Journal of Medicine. 2013–03–14 2013;368(11):1019–1026. https://doi.org/10.1056/nejmoa1200657
Thompson LE, Chan PS, Tang F, et al. Long-Term Survival Trends of Medicare Patients After In-Hospital Cardiac Arrest: Insights from Get With The Guidelines-Resuscitation ®. Resuscitation. 2018–02–01 2018;123:58–64. https://doi.org/10.1016/j.resuscitation.2017.10.023
Xu P, Li D, He Y, et al. Clinical Frailty Scale is more likely to be related to hospital mortality for frail patients suffering in-hospital cardiac arrest. Resuscitation. 2020–03–01 2020;148:215–217. https://doi.org/10.1016/j.resuscitation.2020.01.023
Greenwald JL, Greer JA, Gace D, et al. Implementing Automated Triggers to Identify Hospitalized Patients with Possible Unmet Palliative Needs: Assessing the Impact of This Systems Approach on Clinicians. Journal of Palliative Medicine. 2020–11–01 2020;23(11):1500–1506. https://doi.org/10.1089/jpm.2020.0161
Ariadne Labs: Serious Illness Care. Accessed June 16 2021, https://www.ariadnelabs.org/areas-of-work/serious-illness-care/
Shears M, Takaoka A, Rochwerg B, et al. Assessing frailty in the intensive care unit: A reliability and validity study. J Crit Care. Jun 2018;45:197-203. https://doi.org/10.1016/j.jcrc.2018.02.004
Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. Mar 2001;56(3):M146-56. https://doi.org/10.1093/gerona/56.3.m146
Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. Mar 2013;12(2):719-36. https://doi.org/10.1016/j.arr.2012.03.001
Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. Jun 2010;210(6):901-8. https://doi.org/10.1016/j.jamcollsurg.2010.01.028
Muscedere J, Waters B, Varambally A, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. Aug 2017;43(8):1105-1122. https://doi.org/10.1007/s00134-017-4867-0
Hatcher VH, Galet C, Lilienthal M, Skeete DA, Romanowski KS. Association of Clinical Frailty Scores With Hospital Readmission for Falls After Index Admission for Trauma-Related Injury. JAMA Netw Open. Oct 2 2019;2(10):e1912409. https://doi.org/10.1001/jamanetworkopen.2019.12409
Pranata R, Henrina J, Lim MA, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Archives of gerontology and geriatrics. 2020:104324.
Clayton JM, Butow PN, Arnold RM, Tattersall MH. Discussing life expectancy with terminally ill cancer patients and their carers: a qualitative study. Supportive Care in Cancer. 2005;13(9):733-742.
O'Mara L, Streiter S, Orkaby AR, Ouchi K, Bernacki R. A Framework to Triage Older Adults with Covid-19 to Provide Patient-Centered Care. 2020. Nov 5 2020. doi:10.1056
Acknowledgements
We acknowledge our research assistant, Christina Sheu, BA, for assistance with acquisition of data from the Research Patient Data Registry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Frances Y. Hu and Shoshana Streiter are co-first authors.
Rachelle Bernacki and Ariela Orkaby are co-senior authors.
Rights and permissions
About this article
Cite this article
Hu, F.Y., Streiter, S., O’Mara, L. et al. Frailty and Survival After In-Hospital Cardiopulmonary Resuscitation. J GEN INTERN MED 37, 3554–3561 (2022). https://doi.org/10.1007/s11606-021-07199-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-07199-1