Abstract
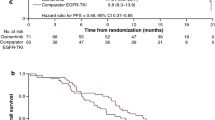
Retrospective studies suggested a benefit of first-line tyrosine kinase inhibitor (TKI) treatment continuation after response evaluation in solid tumors (RECIST) progression in epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC) patients. The aim of this multicenter observational retrospective study was to assess the frequency of this practice and its impact on overall survival (OS). The analysis included advanced EGFR-mutated NSCLC patients treated with first-line TKI who experienced RECIST progression between June 2010 and July 2012. Among the 123 patients included (67 ± 12.7 years, women: 69 %, non smokers: 68 %, PS 0–1: 87 %), 40.6 % continued TKI therapy after RECIST progression. There was no difference between the patients who did and did not continue TKI therapy with respect to progression-free survival (PFS1: 10.5 versus 9.5 months, p = 0.4). Overall survival (OS) showed a non-significant trend in favor of continuing TKI therapy (33.0 vs. 21.2 months, p = 0.054). Progressions were significantly less symptomatic in the TKI continuation group than in the discontinuation group (18 % vs. 37 %, p < 0.01). Univariate analysis showed a higher risk of death among patients with PS >1 (HR 4.33, 95 %CI: 2.21-8.47, p = 0.001), >1 one metastatic site (HR 1.96, 95 %CI: 1.06-3.61, p = 0.02), brain metastasis (HR 1.75, 95 %CI: 1.08-2.84, p = 0.02) at diagnosis, and a trend towards a higher risk of death in cases of TKI discontinuation after progression (HR 1.62, 95 %CI: 0.98-2.67, p = 0.056 ). In multivariate analysis only PS >1 (HR 6.27, 95 %CI: 2.97-13.25, p = 0.00001) and >1 metastatic site (HR 2.54, 95 %CI: 1.24-5.21, p = 0.02) at diagnosis remained significant. This study suggests that under certain circumstances, first-line TKI treatment continuation after RECIST progression is an acceptable option in EGFR-mutated NSCLC patients.
Clinical trial information: NCT02293733


Similar content being viewed by others
References
Barlesi F, Blons-h, Beau-Faller M, et al. Biomarkers (BM) France: Results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol. 2013;31:(suppl; abstr 8000).
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin–paclitaxel inpulmonary adenocarcinoma. N Engl J Med 361:947–957
Zhou C, Wu YL, Cheng G, Feng J, col (2011) Erlotinib versus chemotherapy as first-line treatment forpatients with Advanced EGFR mutation-positive non small cell lung cancer (OPTIMAL, CNTONG-0802):multicentre, open- label, randomised, phase 3 study. Lancet Oncol 12:735–742
Rosell R, Carcereny E, Gervais R, Vergnenegre A et al (2012) Erlotinib versus standard chemotherapy as firstlinetreatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer(EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Gao G, Ren S, Li A et al (2012) Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-linetreatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer 131(5):E822–E829
Sequist LV, Yang JC, Yamamoto N et al (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327–3334
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Choi H, Charnsangavej C, Faria SC et al (2007) correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumortreated at a single institution With Imatinib Mesylate: Proposal of New Computed Tomography Response Criteria. J Clin Oncol 25(13):1753–1759
Benjamin RS, Choi H, Macapinlac HA, v (2007) We Should Desist Using RECIST, at Least in GIST. J Clin Oncol 25:1760–1764
Lee HY, Lee KS, Ahn M-J (2011) New CT response criteria in non-small cell lung cancer: Proposal and application in EGFR tyrosine kinase inhibitor therapy. Lung Cancer 73:63–69
Jackman D, Pao W, Riely GJ et al (2010) clinical definition of acquired resistance toepidermal growth factor receptor tyrosine kinase inhibitors in non–small-cell lung cancer. J Clin Oncol 28:357–360
Weickhardt AJ, Scheier B, Burke JM et al (2012) Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 7(12):1807–1814
Inomata M, Shukuya T, Takahashi T et al (2011) Continuous administration of EGFR-TKIs following radiotherapy after disease progression in bone lesions for non-small cell lung cancer. Anticancer Res 31(12):4519–4523
Nishino M, Cardarella S, Dahlberg SE et al (2013) Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer 79:283–288
Blackwell KL, Burstein HJ, Storniolo AM et al (2010) Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28:1124–1130
Grothey A, Sugrue MM, Purdie DM et al (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26:5326–5334
Extra JM, Antoine EC, Vincent-Salomon A et al (2010) Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist 15:799–809
Conforti F, Catania C, Toffalorio F et al (2013) EGFR tyrosine kinase inhibitors beyond focal progression obtain a prolonged disease control in patients with advanced adenocarcinoma of the lung. Lung Cancer 81:440–444
Nishino K, Imamura F, Morita S et al (2013) A retrospective analysis of 335 Japanese lung cancer patients who responded to initial gefitinib treatment. Lung Cancer 82:299–304
Nishie K, Kawaguchi T, Tamiya A et al (2012) Epidermal Growth Factor ReceptorTyrosine Kinase Inhibitors Beyond Progressive Disease: A Retrospective Analysis for Japanese Patients with Activating EGFR Mutations. J Thorac Oncol 7:1722–1727
Park K, Ahn M, Yu C et al (2014) Aspiration: first-line erlotinib (e) until and beyond recist progression (pd) in asian patients (pts) with egfr mutation-positive(mut+) nsclc. Ann Oncol 25(suppl4):iv426–iv427. doi:10.1093/annonc/mdu349.2
Kim HR, Lee JC, Kim YC, Kim KS et al (2014) Clinical characteristics of non-small cell lung cancer patients who experienced acquired resistance during gefitinib treatment. Lung Cancer 83(2):252–258
Yang JJ, Chen H-J, Yan H-H et al (2013) Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 79(1):33–39
Cortot A, Janne PA (2014) Molecular mechanisms of resistance im epidermal growth factor receptor mutant lung adenocarcinomas. Eur Respir Rev 23:356–366
Chouaid C, Dujon C, Do P et al (2014) Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: A prospective multicenter study in a real-world setting (GFPC study 12–01). Lung Cancer 86:170–173
Mok T, Wu Y, Nakagawa K et al. Gefitinib/chemotherapy vs chemotherapy in epidermal growth factor receptor (EGFR) mutation-positive non-small-cell lung cancer (NSCLC) after progression on first-line gefitinib: The phase III, randomised IMPRESS study. Ann Oncol. 2014;25 (suppl 4). doi 10.1093/annonc/mud438.5
Acknowledgments
This trial was an academic trial conducted by Groupe Français de Pneumo Cancerologie (GFPC).
Compliance with Ethical Standards
ᅟ
Funding
This study received an unrestricted grant from F Hoffmann-La Roche Ltd and Boehringer Ingelheim.
Conflict of Interest
JBA has received personal fees from Boehringer Ingelheim, Hoffman-Roche, Lilly, and Pfizer. CAV has received personal fees from Roche and Astra Zeneca. MP has received personal fees from Roche, Lilly, Astra Zeneca, Pfizer and Bristol Myers Squibb. CDPvH has received personal fees from Roche, Boehringer Ingelheim and Bristol Myers Squibb. SBO has received personal fees from Boehringer and Astra Zeneca. RC and GLG have received personal fees from Hoffman-Roche and Lilly. PF and AV have received personal fees from Boehringer Ingelheim, Hoffman-Roche and Lilly. DA has received personal fees from Hoffman-Roche and Novartis. CC has received personal fees from Astra Zeneca, Boehringer Ingelheim, GlaxoSmithKline, Hoffman-Roche, Sanofi Aventis, Lilly, Novartis, and Amgen. RG has received personal fees from Boehringer Ingelheim, Hoffman-Roche and Astra Zeneca. The other authors (CF, AB, FV, NB, RL, BM) declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Auliac, J.B., Fournier, C., Audigier Valette, C. et al. Impact of Continuing First-Line EGFR Tyrosine Kinase Inhibitor Therapy Beyond RECIST Disease Progression in Patients with Advanced EGFR-Mutated Non-Small-Cell Lung Cancer (NSCLC): Retrospective GFPC 04-13 Study. Targ Oncol 11, 167–174 (2016). https://doi.org/10.1007/s11523-015-0387-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-015-0387-4