Abstract
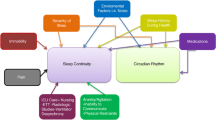
Patients in the acute care units (ACU) are usually critically ill, making them more susceptible to the unfavorable atmosphere in the hospital. One of these unfavorable factors is sleep disruption and deprivation. Many factors may affect sleep in the ACU, including therapeutic interventions, diagnostic procedures, medications, the underlying disease process, and noise generated in the ACU environment. Many detrimental physiological effects can occur secondary to noise and sleep deprivation, including cardiovascular stimulation, increased gastric secretion, pituitary and adrenal stimulation, suppression of the immune system and wound healing, and possible contribution to delirium. Over the past few years, many studies have endeavored to objectively assess sleep in the ACUs, as well as the effect of mechanical ventilation and circadian rhythm changes critically ill patients. At this time, therefore, it is important to review published data regarding sleep in ACUs, in order to improve the knowledge and recognition of this problem by health care professionals. We have therefore reviewed the methods used to assess sleep in ACUs, factors that may affect sleep in the ACU environment, and the clinical implications of sleep disruption in the ACU.
Similar content being viewed by others
Introduction
The acute care units (ACU) in a hospital, which include the medical and surgical intensive care units (MICU and SICU), coronary care units (CCU) and post-anesthesia care units (PACU), are areas for patients who need special attention. Patients in ACUs are usually critically ill, making them more susceptible to the unfavorable atmosphere in the hospital. One of these unfavorable factors is sleep disruption and deprivation [1–4]. Frequently, however, sleep disruption and deprivation is not noticed by the medical staff in ACUs, and many of these professionals have limited knowledge regarding sleep disruption in ACUs and its impact on patient health [5]. In ACUs, many organ systems are aggressively monitored for the development of dysfunction or failure, but frequently the function of sleep in acutely ill patients is ignored.
Many factors may affect sleep in the ACU, including therapeutic interventions, diagnostic procedures, medications, the underlying disease process, and noise generated in the ACU environment. Many detrimental physiological effects can occur secondary to noise, including cardiovascular stimulation, increased gastric secretion, pituitary and adrenal stimulation, suppression of the immune system and wound healing, and possible contribution to delirium [6–10].
It is our opinion that physicians and nurses working in ACUs have a low level of awareness regarding sleep disruption in critically ill patients and the impact of this disruption on the health of these patients. Over the past few years, many studies have endeavored to objectively assess sleep in the ACUs, as well as the effect of mechanical ventilation and circadian rhythm changes in critically ill patients. At this time, therefore, it is important to review published data regarding sleep in ACUs to improve the knowledge and recognition of this problem by health care professionals. We have therefore reviewed the methods used to assess sleep in ACUs, factors that may affect sleep in the ACU environment, and the clinical implications of sleep disruption in the ACU.
Normal sleep
Sleep is a physiological state that humans need to pass through every day to repair and restore body functions. Typically, humans adapt to a 24-h circadian pattern, where they sleep at night and are awake during the day. This 24-h internal clock (circadian pattern) is maintained by environmental factors, primarily light exposure, which affects melatonin secretion at night. Several physiological and biochemical body functions follow this circadian pattern. In normal individuals, sleep latency usually lasts about 10–20 min and sleep lasts for 6–9 h on average, although variability among individuals can be significant.
While asleep, an individual passes through four to six cycles, each of which consists of five sleep stages (Fig. 1b). One is called rapid eye movement (REM), and the rest (stages 1 through 4) are non-REM (NREM) sleep. Sleep onset begins with stage 1, which is a transitional stage, usually about 5% of normal sleep, and then progresses into stage 2, which can constitute as much as 50–60% of sleep. Thereafter, sleep progresses to stages 3 and 4, which are known as delta, slow-wave, or deep sleep. These stages occur predominantly during the first half of the night and constitute 15–20% of night sleep. Compared with light sleep, slow-wave sleep is usually deeper and more restful and requires a more intense stimulus to awaken the sleeper. REM sleep occurs approximately every 90 min in 4–6 cycles, increasing in duration during the second half of the night. During REM sleep, there is inhibition of spinal motor neurons, which leads to paralysis of the major muscle groups but spares the diaphragm and ocular muscles. Although brain activity is increased during REM sleep, this stage is considered to be restful, with a variable arousal threshold. Factors disturbing sleep, such as noise, may affect the integrity of sleep. This may result in electroencephalographic (EEG) arousals and awakenings, which may affect sleep architecture and prevent the normal progress into the deeper stages of sleep [11].
Hypnogram of a patient 2 days after acute myocardial infarction (a) and 6 months later (b). The vertical axis represents sleep stages and the horizontal axis time. a Significant sleep disruptions and awakenings and absence of slow-wave and REM sleep. b Clearly defined sleep cycles with progression to different sleep stages. S1 Stage 1, S2 stage 2, S3 stage 3, S4 stage 4, REM rapid eye movement, MVT movement, WK wake (BaHammam, unpublished data)
Methods for sleep assessment in ACUs
Before discussing factors that affect sleep in ACUs, it is important to describe the methods used to assess sleep in ACU settings. Among the methods used to assess sleep in ACUs are staff observations [12], self-reporting, polysomnography (PSG), and bispectral analysis.
Self-reporting
Several studies have subjectively assessed sleep in ACUs using qualitative and quantitative methodologies. In an evaluation of physical and psychological stressors in 50 intensive care unit (ICU) patients who completed the ICU Environmental Stressor Scale, insomnia was ranked as the second most important stressor, second only to pain [13]. Of patients interviewed 3 days after discharge from the ICU, 61% reported sleep deprivation and 7% rated insomnia as their worst experience in the ICU [14]. In another study, 27% of ICU patients reported having insufficient sleep [15]. Furthermore, perceived ICU sleep quality was poor compared with baseline and did not change over the course of patient stay in the ICU [16]. However, self-reported data suffer from recall bias and a lack of objective assessment of sleep quality.
Polysomnography
The standard diagnostic test for sleep orders is polysomnography, during which neuro-cardio-respiratory parameters are usually monitored. Although PSG studies are usually performed in a sleep laboratory, PSG has been used to assess sleep in critically ill patients in ACUs [2–4], [17–26]. In general, these studies were conducted in different ACUs and involved small groups of patients with different underlying medical and surgical problems, including those with acute coronary syndrome [3, 23], patients recovering from open heart surgery [4], mechanically ventilated patients [22], trauma patients [19], patients with neurological and respiratory disorders [24], and mixed groups of critically ill patients. Previous studies have described the techniques for performing PSG in critically ill patients in ACUs attended by ICU nurses, but not by sleep technicians or in a laboratory setting attended by a sleep technician [23–24]. Overall, sleep recording on ACU patients revealed sleep fragmentation, increases in stages 1 and 2 sleep, reductions in slow-wave and REM sleep, and reductions in total sleep time and sleep efficiency. Most of the previous studies only evaluated sleep during the night, rather than over a 24-h period. However, those studies that did monitor PSG continuously for ≥24 h showed that 40–50% of the total sleep time in ACUs occur during the day [2, 18, 25, 26]. This disturbed sleep pattern may not improve over the course of a patient’s stay in the ICU and may take several days to normalize after discharge from the ICU [21]. For example, repetitive whole-night in-hospital EEG showed that normal sleep patterns did not return to patients with acute myocardial infarction until 9 days after discharge from the ICU [3].
Many factors in the ACUs may disrupt the typical EEG architecture seen during normal sleep, making the interpretation of sleep quality difficult. In a recent study, 24-h PSG monitoring of critically ill mechanically ventilated patients in the ICU revealed that eight patients had disturbed sleep architecture [22]. The remaining patients had either coma (seven patients) or atypical sleep (five patients), in which their EEGs were intermediate between sleep and coma and characterized by a virtual absence of stage 2 and REM sleep [22]. The authors thought that these EEG changes were likely due to sedation.
The bispectral index (BIS)
Because of the difficulties of performing full PSG in the ACUs environment and the need for certain expertise, an alternative method, the bispectral index (BIS), has been used to objectively assess sleep in ACU settings. Previously, the BIS was used extensively by anesthetists to measure the depth of anesthesia and sedation. The BIS monitor displays a real-time EEG trace, acquired from a frontotemporal montage. It combines power spectral analysis with interrogation of the phase relationship between component waves of different frequencies. The monitor generates a dimensionless number on a continuous scale of 0–100, with 100 representing normal cortical electrical activity and 0 indicating cortical electrical silence [27].
Several studies have used the BIS to assess sleep in ACUs (Fig. 2). In one study, the investigators compared the changes in the BIS with the conventional EEG stages of sleep in five subjects during the early part of the night. They reported a good correlation between the BIS and the level of natural sleep [28]. Light sleep occurred at BIS values of 75–90, slow-wave sleep occurred at BIS values of 20–70, and rapid eye movement sleep occurred at BIS values of 75–92 [28]. In another study, which assessed sleep patterns in 27 ICU patients using the BIS and submental electromyogram, none of the patients showed a completely normal sleep pattern [29]. In comparing the BIS and spectral edge frequency (SEF) with PSG in ten patients with mild apnea/hypopnea syndrome to test whether these signals could predict physiologic sleep stages, neither the BIS nor SEF reliably indicated conventionally determined sleep stages [30]. Further studies are needed to access the utility of BIS as a monitor of sleep in patients in ACUs.
Normal changes in sleep stage and BIS in a sleeping healthy volunteer throughout the night (−1, REM sleep; 0, awake; 1 and 2, light sleep; 3 and 4, slow-wave sleep) [29]. Reproduced with permission from the publisher
Skin potential levels of critically ill patients in the ICU have been recorded to monitor the sleep-wake cycle of these patients [31]. Recently, actigraphy, a portable device that records movement over extended periods of time and worn most commonly on the wrist, [32] has been used to assess agitation and sleep in critically ill patients [33, 34]. In one study, actigraphy correlated well with observed and subjective scores on agitation and sedation scales [34]. Further studies are needed to assess the usefulness of this technique in assessing circadian patterns in critically ill patients.
Factors affecting sleep in ACUs
Environmental factors
Environmental factors in ACUs include noise produced by phones, pagers, alarms, and staff and light, which may disturb the normal light-dark cycle.
Noise
Several studies have shown that the noise level in ACUs is quite high [17, 35–41]. Excess noise may increase the mistakes by [42, 43] as well as impair the concentration and mental efficiency of the staff [44, 45]. Noise levels in ACUs range from 60 to 84 dB throughout the day and night [36]. As a reference, a busy office has an average noise level of 70 dB, and a pneumatic drill heard from around 15 m away has a noise level of 80 dB [16, 37]. As noise measurements use a logarithmic scale, an increase of 10 dB represents a doubling of the noise level. The US Environmental Protection Agency (EPA) has recommended that hospital noise levels not exceed 45 dB during the day and 35 dB at night [46]. A study in an intermediate respiratory care unit reported a very strong correlation between the number of patient arousals and the number of sound peaks ≥80 dB [17]. In a study of 26 adult patients in a PACU, the mean integrated sound pressure was 67.1 dB [40]. Staff conversation caused 56% of the recorded noise above 65 dB, while other sources of noise, such as alarms, telephones, and nursing care, accounted for less than 10% each [40]. This finding agrees with that of two other studies, both of which reported that staff conversation was one of the most disruptive environmental noises [16, 25]. Synchronous audio, video, and polysomnographic recording in seven patients showed that 20% of the recorded arousals and awakenings were related to noise and 10% to patient care activities, whereas the causes of the remaining 70% were not identified, indicating that other as yet unknown factors are involved [25]. The above findings concur with those obtained by Freedman et al. [26] in a study of 22 critically ill patients. By simultaneously monitoring environmental noise and PSG, they found that environmental noise was responsible for 11.5 and 17% of the arousals and awakenings from sleep, respectively.
Staff-patient interactions
Despite the increased sophistication of monitoring systems in ACUs, which should decrease hands-on manipulations of sleeping patients, there are still frequent and repetitive staff interactions with critically ill patients, thus reducing patient sleep time in ACUs. Several studies have found that the mean number of staff-patient interactions ranged from 40 to 60 per night [47–49]. Using a 24-h PSG monitoring, it was found that there were eight staff-patient interactions per hour of patient sleep, with most of these interactions due to nursing activities, such as wound dressing, adjustment of intravenous drips, and administration of medications [25]. Nurses were found to routinely provide daily baths for patients between 02:00 and 05:00 on 55 of the 147 study nights [48]. Sleep disruptions caused by human interventions/diagnostic tests were found to be significantly different in different ACUs [16]. For example, patients in the MICU perceived interruptions caused by staff interactions and diagnostic tests to be significantly more disruptive to their sleep than did patients in the SICU and the cardiac intermediate care unit. Based on these results, it has been recommended that nocturnal staff-patient interactions be clustered, thus allowing patients uninterrupted periods of sleep [50–52]. In addition, although most of these studies assessed sleep interruptions and staff interactions at night, future studies should assess these variables during both the day and night.
Circadian pattern disturbances
About 40–50% of the total sleep time in ACUs has been found to occur during the day [2, 18]. For example, using a 24-h PSG monitoring, approximately half the sleep of ventilated patients in the ICU occurred during daytime (06:00–22:00), whereas sleep in healthy controls tended to be more nocturnal [25]. Sleep in humans follows a circadian pattern, in which people usually sleep at night and are awake during the day. This circadian pattern, which is coupled to physiologic cycles, is maintained by internal and external cues called Zeitgebers (makers or cues of time). Among these cues, the most important is exposure to light. Disturbances of the light-dark cycle and frequent environmental noises and interventions in the ACUs may disturb the normal circadian rhythm. Measurable markers for this circadian pattern include body temperature and the levels of certain hormones, such as melatonin and cortisol. Body temperature normally follows a circadian pattern, rising during the day and falling at night. In general, a falling body temperature induces sleep, whereas a rising temperature provokes wakefulness. As shown by recording the rectal temperature of 15 patients who spent greater than 1 week in the ICU, there was a marked disruption of circadian rhythms [53]. Similar findings were reported in otherwise healthy patients following cardiac surgery [54].
Melatonin is considered to be the best marker of circadian rhythm [55, 56]. Individual melatonin profiles are highly reproducible and are less subject to masking factors than are other rhythm markers such as core temperature and cortisol level. Melatonin secretion is normally increased at bedtime and remains high until early morning. Several studies have shown that the circadian release of melatonin is disturbed in critically ill patients [57–61]. For example, when melatonin levels in the blood and urine were measured over three consecutive days in eight critically ill patients during deep sedation and mechanical ventilation, the circadian rhythm of melatonin secretion was abolished in all but one patient, and there was no correlation between melatonin levels and levels of sedation [57]. This suggested that the impairment of the circadian release of melatonin may play a role in sleep disruption and delirium in the ICU. When the urine concentrations of the melatonin metabolite 6-sulphatoxy melatonin (6-SMT) and free cortisol were monitored in 16 patients for their entire stay in the ICU, the diurnal rhythms of both hormones were consistently or periodically disturbed in 65–75% of the patients, despite controlling for illumination [58]. Treatment with adrenergic drugs and benzodiazepines increased 6-SMT excretion [58]. In addition, there was a significant correlation between ICU psychosis and an irregular melatonin circadian rhythm [59].
Hyposecretion of melatonin may have detrimental effects on critically ill patients. In animal models, hyposecretion of melatonin has been shown to impair mitochondrial oxidative phosphorylation [62] and the capacity to survive endotoxemia [63, 64]. Moreover, the antioxidant effects of melatonin have been observed to prevent ischemia-induced renal damage [65, 66]. Although some investigators have recommended that melatonin be given to patients in the ICU to induce sleep and to resynchronize their biological clocks [67], there is little data to support that. Treatment with melatonin may also regulate the secretion of growth hormones and prolactin [68]. Future studies should assess the effect of exogenous melatonin administration on sleep and circadian pattern in acutely ill patients.
Medications
Many drugs are used frequently in ACU settings, including hypnotics, narcotics, and ionotropic drugs. However, heavy sedation does not imply good sleep. For example, although the benzodiazepines decrease sleep latency and awakenings and increase sleep duration and efficiency (sleep duration/time in bed), these drugs also significantly reduce slow-wave and REM sleep, increase spindles, increase cortical activity at low doses, and decrease EEG amplitude at high doses [69–71]. Narcotics also suppress deep and REM sleep and increase arousals and stage 1 sleep [72]. However, the effects of benzodiazepines and narcotics on sleep in patients in the ACUs have not been properly investigated.
Many critically ill patients receive intravenous ionotropic drugs, such as dopamine and norepinephrine. Both of these drugs are important central neurotransmitters and are associated with cortical activation, thus possibly increasing arousals. Fortunately, both drugs do not appear to cross the blood-brain barrier under normal conditions [73]. Nevertheless, the administration of epinephrine to patients sedated with propofol increased their sedation score and BIS values [74]. In rats, blood-brain barrier permeability is increased by tumor necrosis factor alpha (TNF-α), which is secreted as part of the sepsis cascade [75]. Additional studies are needed to examine the effects of these drugs on sleep architecture in critically ill patients.
Mechanical ventilation
Mechanical ventilation is an invasive treatment modality that may affect sleep quality in critically ill patients. Among the causes of sleep interruption in ventilated patients are the severity of the illness, nursing interventions for suctioning and other reasons [49], sedation and other medications, mode of ventilation, asynchrony between the patient and the machine [76], and failure of patients to trigger ventilators due to the use of improper settings, which may result in unloading of the respiratory muscles or intrinsic positive end-expiratory pressure [77]. As factors other than mechanical ventilation may affect sleep in this group of patients, it is difficult to assess the effects of mechanical ventilation on sleep. To date, therefore, there have been only a few studies subjectively or objectively evaluating sleep during mechanical ventilation.
In interviews with 158 survivors of critical illness 2 months after discharge, 30% indicated having experienced agony/panic during mechanical ventilation, which was associated with asynchrony between the patient’s breathing and the ventilator [78]. Using PSG, ventilated critically ill patients were found to have as many as 20–63 arousals and awakenings per hour [22, 23, 25, 79]. In general, PSG monitoring of sleep during mechanical ventilation revealed an increase in stage 1 sleep and reductions in slow-wave and REM sleep [22, 25, 26]. As stage 1 occurs throughout the night as a transitional stage of sleep, increases in stage 1 are considered indicators of sleep disruption [11].
Pressure support has been shown to induce central apneas in healthy subjects during sleep, which in turn may cause sleep disruption [80]. In assessing the influence of the mode of ventilation on sleep quality, 11 critically ill patients were randomized to receive at least 2 h each of three ventilator modes: assist-control ventilation, pressure support alone, and pressure support with dead space [81]. Sleep fragmentation was greater during pressure support (79 vs 54 arousals and awakenings per hour) and sleep efficacy was greater during assist-control (75 vs 63%). Six of the 11 patients developed central apneas while on pressure support but not during assist-control ventilation. Central apnea was more common among patients with heart failure. Among patients with central apneas, adding dead space decreased sleep fragmentation (arousals and awakenings per hour). The most important determinant of central apneas was the difference between a patient’s apnea threshold and arterial partial pressure of carbon dioxide (PaCO2) while breathing at rest [81]. Central apneas were more likely to occur in patients with resting PaCO2 values close to their apnea thresholds. Future studies should systematically explore the effect of other modes of ventilation, such as proportional assist ventilation (PAV) [80], on sleep architecture and the development of central apneas.
When the circadian pattern of melatonin, as 6-SMT, was measured in 16 critically ill patients, the diurnal fluctuation in 6-SMT levels was more likely to be disrupted in patients receiving mechanical ventilation [58]. That is, 6-SMT excretion was significantly lower during periods with mechanical ventilation compared to periods without ventilation. This difference was independent of the mode of ventilation (CPAP vs assist-control ventilation) [58].
Underlying acute and chronic illnesses
Underlying medical problems and complaints may also disturb sleep in acutely ill patients. Among these underlying problems are chronic respiratory disorders, neurological disorders, sleep disorders, heart failure, renal impairment, liver impairment, infection, fever, and pain. For example, there was a significant correlation between sleep fragmentation, measured as the sum of arousals and awakenings, and acuity of illness, measured as APACHE II scores [82, 83].
Sleep disruption has been demonstrated in certain groups of acutely ill patients, such as post-myocardial infarction patients [3, 23] (Fig. 1a), postoperative patients [4, 18, 84, 85], and patients with sepsis [61]. In a nocturnal PSG study of the sleep patterns of 12 patients with acute myocardial infarction, both during their acute illness and after discharge to the general ward for 9 days, the initial PSG in the ICU showed significant disruptions of sleep architecture, with a significant increase in wakefulness and a significant reduction in REM sleep [3]. In these patients, however, sleep quality improved gradually over time. In another study, we evaluated nocturnal sleep quality of 20 patients with acute myocardial infarction who were off sedatives and ionotropes for 48 h and under controlled dark-light exposure and daytime nap, within 3 days of the acute event and 6 months later. To avoid the environmental effects of the acute care areas, both studies were performed in the sleep disorders center and a protocol was implemented to minimize staff-patients interaction (unpublished data). The comparison revealed a significant increase in arousal index, spontaneous arousals, stage shifts, REM latency, and wake time and a significant reduction in total sleep time, sleep efficiency, and REM sleep during the acute event, which indicate that factors other than the ACU environment affect sleep quality in patients with acute myocardial infarction. In a group of patients recovering from elective abdominal surgery, nocturnal PSG demonstrated initially increased awakenings and significant reductions in REM and deep sleep, whereas REM rebound was observed on post-operative night 3 [85]. Using 24- to 48-h PSG monitoring in five critically ill patients with sepsis, Freedman et al. [26] demonstrated that those patients showed no evidence of clearly definable sleep or wake states. This finding is consistent with previous studies showing EEG changes in patients with severe sepsis [86, 87]. Measurements of 6-SMT levels every 4 h over a 24-h period in septic sedated ICU patients, nonseptic ICU patients, and healthy controls showed that the amplitude of the circadian fluctuation of 6-SMT was markedly lower in the septic ICU patients compared with the two other groups [61].
It is worth mentioning here that patients with sleep-related breathing disorders such as obstructive sleep apnea and sleep hypoventilation may present with acute respiratory failure, necessitating ICU admission [24, 88–90]. These patients are usually obese and present with hypercapnic respiratory failure that can be misdiagnosed as chronic obstructive lung disease or congestive heart failure. Early diagnosis and institution of proper treatment may improve outcome [24].
Potential implications of sleep deprivation and disruption
The impact of sleep deprivation and arousals in critically ill patients has not been systematically studied, although the effect of sleep deprivation on healthy adults and the effect of arousals on the cardiovascular system in patients with obstructive sleep apnea or periodic breathing have been extrapolated to critically ill patients. Theoretically, the deleterious effects of sleep deprivation and arousals may be more pronounced in critically ill patients, but more data are needed. The immune system has long been regarded as a vulnerable target for sleep deprivation. Cytokines synthesized by the immune system may play a role in normal sleep regulation, by increasing NREM sleep and decreasing REM sleep, and during inflammatory events, an increase in cytokine levels may intensify their effects on sleep regulation [91]. Current evidence suggests that acute and chronic sleep deprivation is associated with decreased proportions of natural killer cells [92], lower antibody titers following influenza virus immunization [93], reduced lymphokine-activated killer activity [94], and reduced interleukin-2 production [94]. Moreover, sleep deprivation may alter endocrine and metabolic functions, altering the normal pattern of cortisol release and contributing to alterations of “glucocorticoid feedback regulation” [95], glucose tolerance, and insulin resistance [96]. In healthy subjects, sleep deprivation is associated with a negative energy balance [97]. Although the effect of sleep deprivation on ventilatory responses in critically ill patients is not known, sleep deprivation per se in ten healthy subjects did not reduce the sensitivity of central chemoreceptors or change resting ventilation or metabolism [98]. In a recent study of cardiovascular autonomic modulation during 36 h of total sleep deprivation in 18 normal subjects, acute sleep deprivation was associated with increased sympathetic and decreased parasympathetic cardiovascular modulation and decreased baroreflex sensitivity [99]. In addition, frequent arousals were associated with elevated catecholamine release and increased blood pressure [100].
Delirium, formerly known as ICU psychosis or the ICU syndrome, is a serious problem facing the staff in ACUs. The incidence of delirium in a cohort of mechanically ventilated patients in medical and coronary ICUs was found to be as high as 81% [101]. In addition, delirium was an independent predictor of higher 6-month mortality and longer hospital stay, even after adjusting for relevant covariates [102]. Sleep deprivation has been suggested as a risk factor for delirium in critically ill patients. Using staff observations, without objective methods, there was a higher prevalence of delirium among sleep-deprived patients [103, 104]. Delirious patients were reported to have irregular patterns of melatonin release [59] and disrupted circadian rhythms, resulting in fragmented sleep-wake cycles and nighttime awakenings [105]. These findings suggest a strong association between delirium and sleep deprivation but do not establish causality. It must therefore be determined whether sleep deprivation is a cause or a symptom of delirium.
Conclusions and future perspectives
Critically ill patients are prone to sleep deprivation, poor sleep quality, and disturbed circadian patterns. The causes of sleep disruption in these patients seem to be multifactorial and include the ACU environment and staff, diagnostic and therapeutic interventions, medications, mechanical ventilation, and the underlying illness. The role and contribution of each of these factors to sleep disruption in critically ill patients need to be clearly delineated. This will assist in the development of evidence-based guidelines regarding sleep-promoting interventions in ACUs. The effects of newer modes of ventilation on sleep architecture should also be explored. Objective methods that are practical and easy to interpret are needed to assess sleep in the ACUs. The use of BIS may prove to be an easy and practical way to assess sleep in critically ill patients, but more research is needed. Staff working in ACUs should be educated about the importance of sleep, the factors that may disturb sleep, and the role of the ACU staff in promoting good sleep in critically ill patients. Finally, the impact of sleep deprivation and disturbances on patients’ stay in ACUs and on outcome should be determined.
References
Adam K, Oswald I (1983) Protein synthesis, bodily renewal and the sleep-wake cycle. Clin Sci 65:561–567
Hilton BA (1976) Quantity and quality of patients’ sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs 1:453–468
Broughton R, Baron R (1978) Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol 45:348–360
Orr WC, Stahl ML (1977) Sleep disturbances after open heart surgery. Am J Cardiol 39:196–201
Christensen M (2005) What knowledge do ICU nurses have with regard to the effects of noise exposure in the intensive care unit? Intensive Crit Care Nurs 21:199–207
Baker CF (1992) Discomfort to environmental noise: heart rate responses of SICU patients. Crit Care Nurs Q 15:75–90
Tomei F, Papaleo B, Baccolo TP, Persechino B, Spabno G, Rosati MV (1994) Noise and gastric secretion. Am J Ind Med 26:367–372
Haynes C (1999) Emergence delirium: a literature review. Br J Theatre Nurs 9:502–510
Mc Carthy DO, Ouimet ME, Daun JM (1992) The effects of noise stress on leukocyte function in rats. Res Nurs Health 15:131–137
Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne BC, Kapoor SC, Icaza E, Orne MT (1994) Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest 93:1930–1939
Carskadon MA, Dement WC (1999) Normal human sleep: an overview. In: Kryger M, Roth T, Dement WC (eds) Principles and practice of sleep medicine. WB Saunders, Philadelphia, pp 15–26
Edwards GB, Schuring LM (1993) Pilot study: validating staff nurses’ observations of sleep and wake states among critically ill patients, using polysomnography. Am J Crit Care 2:125–131
Novaes MA, Aronovich A, Ferraz MB, Knobel E (1997) Stressors in ICU: patients’ evaluation. Intensive Care Med 23:1282–1285
Simini B (1999) Patients’ perceptions of intensive care. Lancet 354:571–572
Granja C, Lopes A, Moreira S, Dias C, Costa-Pereira A, Carneiro A (2005) Patients’ recollections of experiences in the intensive care unit may affect their quality of life. Crit Care 9:R96–R109
Freedman NS, Kotzer N, Schwab RJ (1999) Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med 159:1155–1162
Aaron JN, Carlisle CC, Carscadon MA, Meyer TJ, Hill NS, Millman RP (1996) Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep 19:707–710
Aurell J, Elmqvist D (1985) Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 290:1029–1032
Fontaine DK (1989) Measurement of nocturnal sleep patterns in trauma patients. Heart Lung 18:402–410
Buckle P, Pouliot Z, Millar T, Kerr P, Kryger MH (1992) Polysomnography in acutely ill intensive care unit patients. Chest 102:288–291
Richards KC, Bairnsfather L (1988) A description of night sleep patterns in the critical care unit. Heart Lung 17:35–42
Cooper AB, Thornley KS, Young GB, Slutsky AS (2000) Sleep in critically ill patients requiring mechanical ventilation. Chest 117:809–818
BaHamman A, Al-Mobeireek A, Al-Nozha M, Al-Tahan A, Binsaeed A (2005) Behaviour and time-course of sleep disordered breathing in patients with acute coronary syndromes. Int J Clin Pract 59:874–880
BaHamman A, Syed S, Al-Mughairy A (2005) Sleep-related breathing disorders in obese patients presenting with acute respiratory failure. Respir Med 99:718–725
Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ (2003) Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med 167:708–715
Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ (2001) Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med 163:451–457
Eke Z, Bell K (2004) Depth of anaesthesia. CPD Anaesth 6:140–142
Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M (1999) The bispectral index: a measure of depth of sleep? Anesth Analg 88:659–661
Nicholson T, Patel J, Sleigh JW (2001) Sleep patterns in intensive care unit patients: a study using the bispectral index. Crit Care Resusc 3:86–91
Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A (2002) Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg 94:125–129
Shiihara Y, Nogami T, Chigira M, Tanno Y, Sakai Y, Takahashi S, Kodama M, Kunimoto F (2001) Sleep-wake rhythm during stay in an intensive care unit: A week’s long-term recording of skin potentials. Psychiatry Clin Neurosci 55:279–280
Littner M, Kushida CA, Anderson WM, Bailey D, Berry RB, Davila DG, Hirshkowits M, Kapen S, Kramer M, Loube D, Wise M, Johnson SF (2003) Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep 26:337–341
Redeker NS, Wykpisz E (1999) Pain, fatigue, activity, and sleep after coronary bypass (abstract). Better health through nursing research. State of the Science Congress, American Academy of Nursing, Washington, DC
Grap MJ, Borchers CT, Munro CL, Elswick RK Jr, Sessler CN (2005) Actigraphy in the critically ill: correlation with activity, agitation, and sedation. Am J Crit Care 14:52–60
Falk SA, Woods NF (1973) Hospital noise-levels and potential health hazards. N Engl J Med 289:774–781
Meyer TJ, Eveloff SE, Bauer MS, Schwartz WA, Hill NS, Millman RP (1994) Adverse environmental conditions in the respiratory and medical ICU settings. Chest 105:1211–1216
Redding JS, Hargest TS, Minsky SH (1977) How noisy is intensive care? Crit Care Med 5:275–276
Gowan NJ (1979) The perceptual world of the intensive care unit: an overview of some environmental considerations in the helping relationship. Heart Lung 8:340–344
Soutar RL, Wilson JA (1986) Does hospital noise disturb patients? Br Med J (Clin Res Ed) 292:305
Allaouchiche B, Duflo F, Debon R, Bergeret A, Chassard D (2002) Noise in the postanaesthesia care unit. Br J Anaesth 88:369–373
Monsén MG, Edéll-Gustafsson UM (2005) Noise and sleep disturbance factors before and after implementation of a behavioural modification programme. Intensive Crit Care Nurs 21:208–219
Murthy VS, Malhotra SK, Bala I, Raghunathan M (1995) Detrimental effects of noise on anaesthetists. Can J Anaesth 42:608–611
Balogh D, Kittinger E, Benzer A, Hackl JM (1993) Noise in the ICU. Intensive Care Med 19:343–346
Smith A (1989) A review of the effects of noise on human performance. Scand J Psychol 30:185–206
Park SH, Song HH, Han JH, Park JM, Lee EJ, Park SM, Kang KJ, Lee JH, Hwang SS, Rho SC et al (1994) Effect of noise on the detection of rib fractures by residents. Invest Radiol 29:54–58
Agency UEP (1974) Information on levels of environmental noise requisite to protect the public health and welfare with an adequate margin of safety. US Government Printing Office, Washington, DC
Woods NF (1972) Patterns of sleep in postcardiotomy patients. Nurs Res 21:347–352
Tamburri LM, DiBrienza R, Zozula R, Redeker NS (2004) Nocturnal care interactions with patients in critical care units. Am J Crit Care 13:102–112
Celik S, Öztekin D, Akyolcu N, Ýssever H (2005) Sleep disturbance: the patient care activities applied at the night shift in the intensive care unit. J Clin Nurs 14:102–106
Sakallaris B, Orrel B (1997) Effects of a 6-hour block of uninterrupted time on postcardiac surgery patients’ perceived sleep and pain. Am J Crit Care 6:243 (Abstract)
Edwards JB, Schuring LM (1993) Sleep protocol: a research-based practice change. Crit Care Nurse 13:84–88
Dines-Kalinowski CM (2002) Nature’s nurse: promoting sleep in the ICU. Dimens Crit Care Nurs 21:32–34
Tweedie IE, Bell CF, Clegg A, Campbell IT, Minors DS, Waterhouse JM (1989) Retrospective study of temperature rhythms of intensive care patients. Crit Care Med 17:1159–1165
Nutall GA, Kumar M, Murray MJ (1998) No difference exists in the alteration of circadian rhythm between patients with and without intensive care unit psychosis. Crit Care Med 26:1351–1355
Arendt J (1995) Melatonin and the mammalian pineal gland. Chapman & Hall, London, UK
Miles A, Thomas R (1988) Melatonin—a diagnostic marker in laboratory medicine? In: Miles A, Philbrick DRS, Thompson C (eds) Melatonin: clinical perspectives. Oxford University Press, Oxford, UK, pp 253–279
Olofsson K, Alling C, Lundberg D, Malmros C (2004) Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand 48:679–684
Frisk U, Olsson J, Nylen P, Hahn RG (2004) Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci 107:47–53
Miyazaki T, Kuwano H, Kato H, Ando H, Kimura H, Inose T, Ohno T, Suzuki M, Nakjima M, Manda R, Fukuchi M, Tsukada K (2003) Correlation between serum melatonin circadian rhythm and intensive care unit psychosis after thoracic esophagectomy. Surgery 133:662–668
Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, Balaum H, Shenkman L (1999) Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci 317:278–281
Mundigler G, Delle-Karth G, Koreny M, Zehetgruber M, Steindl-Munda P, Marktl W, Ferti L, Siostrzonek P (2002) Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 30:536–540
Martin M, Macias M, Leon J, Escames G, Khaldy H, Acuna-Castrovieho D (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol 34:348–357
Maestroni GJ (1996) Melatonin as a therapeutic agent in experimental endotoxic shock. J Pineal Res 20:84–89
Escames G, Leon J, Macias M, Khaldy H, Acuna-Castroviejo D (2003) Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. FASEB J 17:932–934
Tunez I, del Carmen Munoz M, Feijoo M, Valdelvira ME, Rafael Munoz-Castaneda J, Montilla P (2003) Melatonin effect on renal oxidative stress under constant light exposure. Cell Biochem Funct 21:35–40
Kunduzova OR, Escourrou G, Seguelas MH, Delagrange P, De La Farge F, Cambon C, Parini A (2003) Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J 17:872–874
Shilo L, Dagan Y, Smorjik Y, Weinberg U, Dolev S, Komptel B, Shenkman L (2000) Effect of melatonin on sleep quality of COPD intensive care patients: a pilot study. Chronobiol Int 17:71–76
Petterborg LJ, Thalen BE, Kjellman BF, Wetterberg L (1991) Effect of melatonin replacement on serum hormone rhythms in a patient lacking endogenous melatonin. Brain Res Bull 27:181–185
Qureshi A, Lee-Chiong T Jr (2004) Medications and their effects on sleep. Med Clin North Am 88:751–766
Borbély AA, Mattmann P, Loepfe M, Strauch I, Lehmann D (1985) Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol 4:189–194
Achermann P, Borbély AA (1987) Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Hum Neurobiol 6:203–210
Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO (2001) Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep 24:39–44
Oldendorf WH (1971) Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol 221:1629–1639
Andrzejowski J, Sleigh JW, Johnson IA, Sikiotis L (2000) The effect of intravenous epinephrine on the bispectral index and sedation. Anaesthesia 55:761–763
Bourne RS, Mills GH (2004) Sleep disruption in critically ill patients—pharmacological considerations. Anaesthesia 59:374–384
Flick GR, Bellamy PE, Simmons DH (1989) Diaphragmatic contraction during assisted mechanical ventilation. Chest 96:130–135
Hubmayr RD (1994) Setting the ventilator. In: Tobin MI (ed) Principles and practice of mechanical ventilation. McGraw-Hill, New York, pp 191–206
Bergbom-Engberg I, Haljamae H (1989) Assessment of patients’ experience of discomforts during respirator therapy. Crit Care Med 17:1068–1072
Gottschlich MM, Jenkins ME, Mayes T, Khoury J, Kramer M, Warden GD, Kagan RJ (1994) The 1994 Clinical Research Award. A prospective clinical study of the polysomnographic stages of sleep after burn injury. J Burn Care Rehabil 15:486–492
Meza S, Mendez M, Ostrowski M, Younes M (1998) Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol 85:1929–1940
Partasarathy S, Tobin MJ (2002) Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med 166:1423–1429
Parthasarathy S, Tobin MJ (2003) Is sleep disruption related to severity of critical illness? Am J Respir Crit Care Med 167:A968 (Abstract)
Parthasarathy S (2004) Sleep during mechanical ventilation. Curr Opin Pulm Med 10:489–494
Kavey NB, Altshuler KZ (1983) Flurazepam and the sleep of herniorrhaphy patients. J Clin Pharmacol 23:199–208
Knill RL, Moote CA, Skinner ML, Rose EA (1990) Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology 73:52–61
Bolton CF, Young GB, Zochodne DW (1993) The neurological complications of sepsis. Ann Neurol 33:94–100
Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA (1990) The encephalopathy associated with septic illness. Clin Invest Med 13:297–304
Fletcher EC, Shah A, Qian W, Miller CC (1991) “Near miss” death in obstructive sleep apnea: a critical care syndrome. Crit Care Med 19:1158–1164
Buckle P, Pouliot Z, Millar T, Kerr P, Kryger MH (1992) Polysomnography in acutely ill intensive care unit patients. Chest 102:288–291
Resta O, Guido P, Foschimo Barbaro MP, Picca V, Talamo S, Lamorgese P (2000) Sleep-related breathing disorders in acute respiratory failure assisted by non-invasive ventilatory treatment: utility of portable polysomnographic system. Respir Med 94:128–134
Krueger JM, Majde JA (2003) Humoral links between sleep and the immune system: research issues. Ann N Y Acad Sci 992:9–20
Osturk L, Pelin Z, Van Cauter E (1999) Effects of 48 hours sleep deprivation on human immune profile. Sleep Res Online 2:107–111
Spiegel K, Sheridan JF, Van Cauter E (2002) Effect of sleep deprivation on response to immunization. JAMA 288:1471–1472
Irwin M, McClintick C, Costlow C, Fortner M, White J, Gillin JC (1996) Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J 10:643–653
Ledproult R, Copinschi G, Buxton O, Van Cauter E (1997) Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 20:865–870
Speigel K, Leproult R, Van Cauter E (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354:1435–1439
Scrimshaw NS, Habicht JP, Pellet P, Piche ML, Cholakos B (1966) Effects of sleep deprivation and reversal of diurnal activity on protein metabolism of young men. Am J Clin Nutr 19:313–319
Spengler CM, Shea SA (2000) Sleep deprivation per se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med 161:1124–1128
Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MHN, Demeersman RE, Basner RC (2005) Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol 98:2024–2032
Loredo JS, Zeigler MG, Ancoli-Israel S, Clausen JL (1999) Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest 116:655–659
Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK (2001) The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med 27:1892–1900
Ely EW, Shintani A, Truman B, Speroff T, Gordon SSSM, Harrell FE Jr, Inouye SK, Bernard GR, Dittus RS (2004) Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 291:1753–1762
Heller SS, Frank KA, Malm JR, Bowman FO Jr, Harris PD, Charlton MH, Kornfeld DS (1970) Psychiatric complications of open-heart surgery: a re-examination. N Engl J Med 283:1015–1020
Helton MC, Gordon SH, Nunnery SL (1980) The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung 9:464–468
Mira S, GanZini L (2003) Delirium, depression, and anxiety. Crit Care Clin 19:771–787
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
BaHammam, A. Sleep in acute care units. Sleep Breath 10, 6–15 (2006). https://doi.org/10.1007/s11325-005-0044-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-005-0044-8