Abstract
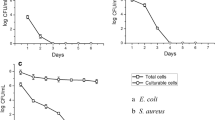
With the growing microbial resistance to conventional antimicrobial agents, the development of novel and alternative therapeutic strategies are vital. During recent years novel peptide antibiotics with broad spectrum activity against many Gram-positive and Gram-negative bacteria have been developed. In this study, antibacterial activity of CM11 peptide (WKLFKKILKVL-NH2), a short cecropin–melittin hybrid peptide, is evaluated against antibiotic-resistant strains of Klebsiella pneumoniae and Salmonella typhimurium as two important pathogenic bacteria. To appraise the antibacterial activity, minimal inhibitory concentration (MIC), minimal bactericidal concentration (MBC) and bactericidal killing assay were utilized with different concentrations (2–128 mg/L) of peptide. To evaluate cytotoxic effect of peptide, viability of RAJI, Hela, SP2/0, CHO, LNCAP cell lines and primary murine macrophage cells were also investigated with MTT assay in different concentrations (3–24 and 0.5–16 mg/L, respectively). MICs of K. pneumoniae and S. typhimurium isolates were in range of 8–16 and 4–16 mg/L, respectively. In bactericidal killing assay no colonies were observed at 2X MIC for K. pneumoniae and S. typhimurium isolates after 80–90 min, respectively. Despite the fact that CM11 reveals no significant cytotoxicity on RAJI, Hela, SP2/0, and CHO cell lines beneath 6 mg/L at first 24 and 48 h, the viability of LNCAP cells are about 50 % at 3 mg/L, which indicates strong cytotoxicity of the peptide. In addition, macrophage toxicity by MTT assay showed that LD50 of CM11 peptide is 12 μM (16 mg/L) after 48 h while in this concentration after 24 h macrophage viability was about 70 %.




Similar content being viewed by others
References
Akhtar MS, Nadeem MA, Shahid A (2011) Antimicrobial peptides as infection imaging agents: better than radiolabeled antibiotics. Int J Pept 2012:1–19
Andreu D, Ubach J, Boman A, Wahlin B, Wade D, Merrifield RB, Boman HG (1992) Shortened cecropin A–melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett 296(2):190–194
Andrew MRW (2009) Antimicrobial resistance update: Klebsiella pneumoniae carbapenemases. Drug Benefit Trends 21(8)
Badosa E, Ferre R, Planas M, Feliu L, Besalu E, Cabrefiga J, Bardaji E, Montesinos E (2007) A library of linear undecapeptides with bactericidal activity against phytopathogenic bacteria. Peptides 28(12):2276–2285. doi:10.1016/j.peptides.2007.09.010
Baliga K (2007) Drug resistance in Salmonella typhi: tip of the Iceberg. Online J Health 3(4):1–2
Bhargava K, Feix JB (2004) Membrane binding, structure, and localization of cecropin–mellitin hybrid peptides: a site-directed spin-labeling study. Biophys J 86(1 Pt 1):329–336. doi:10.1016/S0006-3495(04)74108-9
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Rev Microbiol 3(3):238–250. doi:10.1038/nrmicro1098
Brown KL, Hancock RE (2006) Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 18(1):24–30. doi:10.1016/j.coi.2005.11.004
Butu MB (2011) Antimicrobial peptides—natural antibiotics. Roman Biotech Lett 16(3):6135–6145
Cao Y, Yu RQ, Liu Y, Zhou HX, Song LL, Qiao DR (2010) Design, recombinant expression, and antibacterial activity of the cecropins–melittin hybrid antimicrobial peptides. Curr Microbiol 61(3):169–175. doi:10.1007/s00284-010-9592-7
Cavallarin L, Andreu D, Segundo BS (1998) Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol Plant Microbe Interact 11(3):218–227. doi:10.1094/Mpmi.1998.11.3.218
Cederlund A, Gudmundsson GH, Agerberth B (2011) Antimicrobial peptides important in innate immunity. FEBS J 278(20):3942–3951. doi:10.1111/j.1742-4658.2011.08302.x
Chou HT, Wen HW, Kuo TY, Lin CC, Chen WJ (2010) Interaction of cationic antimicrobial peptides with phospholipid vesicles and their antibacterial activity. Peptides 31(10):1811–1820. doi:10.1016/j.peptides.2010.06.021
Christensen B, Fink J, Merrifield RB, Mauzerall D (1988) Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA 85(14):5072–5076
Epand RM, Vogel HJ (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta 1462(1–2):11–28
Ferre R, Badosa E, Feliu L, Planas M, Montesinos E, Bardaji E (2006) Inhibition of plant-pathogenic bacteria by short synthetic cecropin A–melittin hybrid peptides. Appl Environ Microbiol 72(5):3302–3308. doi:10.1128/Aem.72.5.3302-3308.2006
Giacometti A, Cirioni O, Kamysz W, D’Amato G, Silvestri C, Simona Del Prete M, Lukasiak J, Scalise G (2004) In vitro activity and killing effect of the synthetic hybrid cecropin A-melittin peptide CA(1–7)M(2–9)NH(2) on methicillin-resistant nosocomial isolates of Staphylococcus aureus and interactions with clinically used antibiotics. Diagn Microbiol Infect Dis 49(3):197–200
Gordon YJ, Romanowski EG, McDermott AM (2005) A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30(7):505–515
Hancock RE, Patrzykat A (2002) Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord 2(1):79–83
Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F (2012) Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113(4):723–736. doi:10.1111/j.1365-2672.2012.05338.x
Heather MK, Feix JB (2011) Effects of D-lysine substitutions on the activity and selectivity of antimicrobial peptide CM15. Polymers-Basel 2011(3):2088–2106
Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G (2009) Epidemic multiple drug resistant Salmonella typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19(12):2279–2287. doi:10.1101/gr.091017.109
Lee MT, Hung WC, Chen FY, Huang HW (2008) Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci USA 105(13):5087–5092. doi:10.1073/pnas.0710625105
Leegaard TM, Caugnat DA, Froholm LO, Hoiby EA, Lassen J (2000) Emerging antibiotic resistance in Salmonella typhimurium in Norway. Epidemiol Infect 125(3):473–480
Madani F, Lindberg S, Langel U, Futaki S, Graslund A (2011) Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011:414729. doi:10.1155/2011/414729
Montesinos E (2007) Antimicrobial peptides and plant disease control. FEMS Microbiol Lett 270(1):1–11. doi:10.1111/j.1574-6968.2007.00683.x
Moosazadeh MM, Abolhasani F, Babavalian H, Mirnejad R, Azizi KB, Amani J (2012) Comparison of in vitro antibacterial activities of two cationic peptides CM15 and CM11 against five pathogenic bacteria: Pseudomonas aeruginosa, Staphylococcus aureus, Vibrio cholerae, Acinetobacter baumannii, and Escherichia coli. Probiotics Antimicrob Proteins 4(2):135–139
Munoz-Price LS, Hayden MK, Lolans K, Won S, Calvert K, Lin M, Stemer A, Weinstein RA (2010) Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect Control Hosp Epidemiol 31(4):341–347. doi:10.1086/651097
National Committee for Clinical Laboratory Standards (NCCLS) (2010) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6
Pacor S, Giangaspero A, Bacac M, Sava G, Tossi A (2002) Analysis of the cytotoxicity of synthetic antimicrobial peptides on mouse leucocytes: implications for systemic use. J Antimicrob Chemother 50(3):339–348
Papo N, Shai Y (2005) Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci 62(7–8):784–790. doi:10.1007/s00018-005-4560-2
Park SC, Park Y, Hahm KS (2011) The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci 12(9):5971–5992. doi:10.3390/Ijms12095971
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP (2008) Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29(12):1099–1106. doi:10.1086/592412
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11(4):589–603
Rodriguez-Hernandez MJ, Saugar J, Docobo-Perez F, de La Torre BG, Pachon-Ibanez ME, Garcia-Curiel A, Fernandez-Cuenca F, Andreu D, Rivas L, Pachon J (2006) Studies on the antimicrobial activity of cecropin A–melittin hybrid peptides in colistin-resistant clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother 58(1):95–100. doi:10.1093/Jac/Dkl145
Rudenko SV, Madanat WKM (2005) Modulatory effect of peptide structure and equilibration conditions of cell on peptide-induced hemolysis. Bull Kharkiv Karazin Natl Univ Ser Biol 709(1–2):139–146
Ryadnov MG, Degtyareva OV, Kashparov IA, Mitin YV (2002) A new synthetic all-D-peptide with high bacterial and low mammalian cytotoxicity. Peptides 23(10):1869–1871
Saugar JM, Rodriguez-Hernandez MJ, de la Torre BG, Pachon-Ibanez ME, Fernandez-Reyes M, Andreu D, Pachon J, Rivas L (2006) Activity of cecropin A–melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother 50(4):1251–1256. doi:10.1128/Aac.50.4.1251-1256.2006
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66(4):236–248. doi:10.1002/Bip.10260
Sverre LS, Nissen-Meyer J, Sand O, Haug TM (2013) Plantaricin A, a cationic peptide produced by Lactobacillus plantarum, permeabilizes eukaryotic cell membranes by a mechanism dependent on negative surface charge linked to glycosylated membrane proteins. Biochim Biophys Acta 1828(2):249–259
Vaucher RA, Teixeira ML, Brandelli A (2010) Investigation of the cytotoxicity of antimicrobial peptide P40 on eukaryotic cells. Curr Microbiol 60(1):1–5. doi:10.1007/s00284-009-9490-z
Wiesner J, Vilcinskas A (2010) Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1(5):440–464. doi:10.4161/viru.1.5.12983
Wilcox S (2004) The new antimicrobials: cationic peptides. Bio Teach J 2(1):88–91
Yeagle PL (1985) Cholesterol and the cell membrane. Biochim Biophys Acta 822(3–4):267–287
Zasloff M (2002a) Antimicrobial peptides in health and disease. New Engl J Med 347(15):1199–1200. doi:10.1056/Nejme020106
Zasloff M (2002b) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395. doi:10.1038/415389a
Zasloff M (2002c) Innate immunity, antimicrobial peptides, and protection of the oral cavity. Lancet 360(9340):1116–1117. doi:10.1016/S0140-6736(02)11239-6
Zhang L, Falla TJ (2006) Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother 7(6):653–663. doi:10.1517/14656566.7.6.653
Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. In: Coligan JE et al (eds) Current protocols in immunology, Chapter 14:Unit 14 11. doi:10.1002/0471142735.im1401s83
Acknowledgments
This study was supported and conducted by Applied Biotechnology Research Center. The authors like to thanks Dr. Ali Mohammad Latifi for his kind and generous assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moghaddam, M.M., Barjini, K.A., Ramandi, M.F. et al. Investigation of the antibacterial activity of a short cationic peptide against multidrug-resistant Klebsiella pneumoniae and Salmonella typhimurium strains and its cytotoxicity on eukaryotic cells. World J Microbiol Biotechnol 30, 1533–1540 (2014). https://doi.org/10.1007/s11274-013-1575-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1575-y