Abstract
Introduction
Life expectancy for people with spinal cord injury has shown a marked increase due to modern advances in treatment methods and in neuro-urology. However, since life expectancy of people with paralysis increases, the risk of developing of urinary bladder cancer is gaining importance.
Materials and methods
Single-centre retrospective evaluation of patient data with spinal cord injuries and proven urinary bladder cancer and summary of the literature.
Results
Between 1998 and 2014, 24 (3 female, 21 male) out of a total of 6599 patients with spinal cord injury were diagnosed with bladder cancer. The average age at bladder cancer diagnosis was 57.67 years, which is well below the average for bladder cancer cases in the general population (male: 73, female: 77). All but one patient had a latency period between the onset of the spinal paralysis and tumour diagnosis of more than 10 years. The median latency was 29.83 years. The median survival for these patients was 11.5 months. Of the 24 patients, 19 (79%) had muscle invasive bladder cancer at ≥T2 at the time of diagnosis. The type of neurogenic bladder (neurogenic detrusor overactivity or acontractility) and the form of bladder drainage do not appear to influence the risk. Long-term indwelling catheter drainage played only a minor role in the investigated patients.
Conclusions
The significantly younger age at onset and the frequency of invasive tumours at diagnosis indicate that spinal cord injury influences bladder cancer risk and prognosis as well. Early detection of bladder cancer in patients with spinal cord injury remains a challenge.
Similar content being viewed by others
Introduction
Urinary bladder cancer is the 5th most common cancer in men and the 17th most common cancer in women [1] worldwide. Main risk factors for bladder cancer are tobacco smoking, associated with about 50% of all bladder cancer cases in men and women [2], and exposure to occupational carcinogens, associated with 7.1% of the cases in men and 1.9% of the cases in women [3]. Bladder cancer in patients with spinal cord injury (SCI) was subject of up to now only 17 published studies [4–20]. It was concluded that SCI may be a risk factor for urinary bladder cancer. As the life expectancy of people with SCI is improving due to medical advances in the management of paraplegia and neuro-urology [21, 22], this issue is likely to become increasingly important. However, the pathomechanism leading to an increased bladder cancer risk in patients with SCI is unknown. Further, no criteria are available to compensate bladder cancer in SCI patients as a sequela of the injury.
The objective of this study is to present information that allows to compensate bladder cancer cases as a sequela of a spinal cord injury, based on patient database analysis at the Centre for Spinal Cord Injuries of the BG Trauma Hospital Hamburg in association with data from the relevant literature, and to demonstrate the need for an early detection of the disease.
Materials and methods
The patient database of the Centre for Spinal Cord Injuries at BG Trauma Hospital Hamburg was retrospectively searched to identify patients with SCI who developed bladder cancer in the period between January 1998 and December 2014. The data records of 6599 SCI patients were evaluated. Patient characteristics, data of spinal cord injury, tumour characteristics, bladder management, risk factors, tumour treatment and outcome data were extracted from patient charts. The study included all consecutive SCI patients presented to our institution as inpatient or outpatient between January 1998 and December 2014. There was no screening strategy for bladder tumours in our institution, so the tumours were diagnosed during regular “check-up”-procedures (sonographically or cystoscopically) or if they became symptomatic, with haematuria, hydronephrosis or frequently recurrent urinary tract infections. There was no regular follow-up of tumour patients, but all SCI patients were included in our system of “life-long surveillance”. Therefore, in fact, the follow-up time is different and depends on the date of SCI (entrance in our clinic) in the study period.
Moreover, a selective literature search was performed using MEDLINE/PubMed database on the search items “bladder cancer AND spinal cord injury”. The search was performed up to October 31, 2015. The search revealed 184 hits, thereof 60 case reports and 23 reviews. After exclusion of papers not presenting the required original data on SCI to answer our questions, 17 studies remained for evaluation. References known to the authors or cited in the searched literature were also used. Medians and means including standard deviation were calculated using Microsoft™ Excel™.
All applicable institutional and governmental regulations concerning the ethical use of the data were observed. The approving institutional review board was the Institution for Statutory Accident Insurance and Prevention in the Health and Welfare Services (address: Pappelallee 33, 22089 Hamburg, date June 22, 2015).
Results
Hamburg data
Totally, 24 spinal cord injured patients with bladder cancer were identified (Table 1). A superficial carcinoma of the urinary bladder was detected in three more patients during their initial treatment after the onset of paralysis. These patients were excluded from further analysis.
The simple incidence rate of bladder cancer in a total number of paraplegic patients treated over the study period at BG Trauma Hospital Hamburg was calculated as 0.36%.
Three of the cancer patients were women, and 21 were men (Fig. 1). Median age at diagnosis of the tumour for the whole group was 54.5 years (mean 57.67, standard deviation 11.83 years), for men 55.0 years (mean 57.71, standard deviation 11.93 years) and for women 51.0 years (mean 57.33, standard deviation 13.65 years). Median age at diagnosis for the SCI patients was lower for men (by 18.5 years) than for the general population, for women it was no less than 26 years lower (Robert Koch Institute (RKI) data 2010: Median men 73, women 77 years [23]).
Histology results showed 19 urothelial cell carcinoma (transitional cell carcinoma—TCC, median age 54.0, mean 56.58 years, standard deviation 11.91 years), four squamous cell carcinoma (SCC, median age 67.5, mean 65.00 years, standard deviation 10.80 years) and one completely undifferentiated carcinoma. The proportion of SCC was thus 16.7%. The relative incidence of squamous cell carcinoma in the general population is presumed to be 3–6.8% [24, 25]. It is worthy of note that the patients diagnosed with squamous cell carcinoma were 8.5 years older on average (mean values) than those with urothelial cell carcinoma.
Median latency (Fig. 2) between the onset of paralysis and discovery of the tumour was 31.5 years (mean 29.83 years, standard deviation 11.39 years), 29.0 for TCC (mean 27.31, standard deviation 10.33 years) and for SCC 36.50 years (mean 35.75, standard deviation 13.4 years). Median latency for SCC was thus 7.5 years longer than for TCC. The shortest time span between the onset of paraplegia and bladder cancer (TCC) was 8 years in one male patient.
The distribution of SCI levels is shown in Fig. 3. A total of 21 out of 24 patients had a lesion of the upper motor neuron with neurogenic detrusor overactivity, while three patients whose level of SCI was below L1 had lower motor neuron lesion with acontractile detrusor.
The course of the disease was followed in all 24 patients. The prognosis was poor. Twelve of the 24 patients had died of their cancer after 1 year (Fig. 4). All the deaths were tumour-associated. Median survival for the whole group was 11.5 months (mean 22.71 months, standard deviation 30.42 months). This means that the prognosis for SCI patients with bladder cancer is drastically worse than for the general population (RKI: absolute 5-year survival rate (2009–2010) men 47%, women 41% [23])—even though most of these patients had been monitored (neuro)urologically on a regular basis due to their neurogenic lower urinary tract dysfunction.
Nor did patients do much better after radical cystectomy—their median survival was 15.0 months (range 2–106 months, mean 27.47 months, standard deviation 29.15 months).
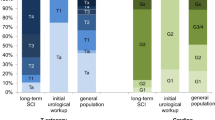
This substantially poorer prognosis matches an unfavourable tumour category: 19/24 patients were staged T2 or higher with invasion of the muscle wall (15/19 TCC, 3/4 SCC and 1 undifferentiated carcinoma). The others were two T1 tumours (both TCC and poorly differentiated (G3) and three pTa tumours (2 × G1 (1 × TCC, 1 × SCC), 1 × G2 (TCC)). The only two long-term survivors (currently 101 and 106 months) were one with a papillary pTaG1 urothelial carcinoma, in which the tumour had been incidentally discovered during a cystoscopy prior to a botulinum A toxin injection in the detrusor muscle, and one patient with a radically resected pT4a urothelial cell carcinoma. The incidence of invasive urothelial cell carcinoma at initial diagnosis is about 20% in the general population (whereas invasive SCC accounts for around 80%) [26].
The type of bladder drainage was also recorded (Table 1). The great majority of patients drained the bladder by means of reflex micturition or intermittent catheterisation. In a total latency of 8592 months, only 167 “indwelling catheter months” (1.94%) were found. Interestingly, the indwelling catheter rate was even lower in patients with SCC: 2 out of a total of 1716 months of latency (0.12%) compared with TCC patients: 165 out of a total of 6540 months of latency (2.55%).
A total of 15 out of 24 patients underwent radical cystectomy (plus 4 palliative transurethral bladder tumour resection (TUR-B), 2 palliative chemotherapy, each with TUR-B with re-TUR-B, palliative ureterocutaneostomy (UCN) without cystectomy and palliative treatment only). Of the cystectomies, drainage in twelve cases was by ileum conduit, two in the form of a Mainz pouch I (both women with TCC) and one UCN.
Only 5/24 patients had a previous history of bladder stones. 10/24 patients had suffered recurrent urinary tract infections (3 or more episodes per year). No valid details of smoking were documented for 16 out of 24 patients in the Hamburg database.
Discussion
Incidence of urinary bladder cancer in SCI patients
The figures for the incidence of bladder cancer in patients with SCI in the literature are heterogeneous and partly debatable. Whereas in some mainly older studies [4, 5, 8, 12] an excessively high risk was reported, later studies show lower but still significantly above-average disease rates compared with the general population. However, it is worthy of note that most studies give only simple incidence rates with very varied follow-up and do not estimate age- or gender-specific rates. The raw incidence figures in more recent studies fluctuate between 0.11 and 2.43%. In an older, small study by Kaufman et al. [5], it was as high as 9.58% (Table 2).
Subramonian et al. [17] gave an age-standardised incidence of 30.7 per 100,000 person years (95% CI 0.4–61.1). In the population at large in the same region, the incidence standardised against the European general population was 14.4/100,000 person years (95% CI 13.9–14.9). This difference was not significant, however.
Groah et al. [14] calculated the age-adjusted incidence rate in 3670 SCI patients at 18.6/100,000 person years (for patients without indwelling catheters) and up to 77/100,000 person years for patients with indwelling catheter. When adjusted by age and sex, the morbidity rate was 15.2 times higher (95% CI 9.2–23.3) than for the general population.
A population-based study [29] of 54,401 SCI patients from Taiwan showed a lower incidence of urinary bladder cancer in patients with SCI (2.56/100,000 person years) than in the population for comparison (2.82/100,000 person years), although it was higher in under-fifties (adjusted hazard ratio 1.28, 95% CI 0.64–2.59).
In contrast, another recent population-based study from Taiwan found a bladder cancer incidence rate of 68.90 per 100,000 person years in 1816 SCI patients with a maximum follow-up of 11 years [30]. The SCI patients had a bladder cancer incidence that was 5.74 times greater than the incidence in a healthy control group. The adjusted hazard ratio was 6.51 (95% CI 2.56–16.52, p < 0.001). Interestingly, the overall bladder cancer risk was not significantly different between the SCI patients and a comparable group of non-SCI-patients with indwelling catheters (adjusted hazard rate 9.11, 95% CI 3.90–21.29, p < 0.001).
By comparison, the Robert Koch Institute sets the age-standardised (against the European population) incidence rate of bladder cancer in 2011 at 17.8/100,000 for male and 5.2/100,000 for female inhabitants of Germany [31]. The “GLOBOCAN-Project” by the International Agency for Research on Cancer (IARC) which is part of the WHO [1] estimates the age-standardised incidence of bladder cancer for both sexes in Germany for 2012 at 13.4/100,000 inhabitants (men 22.7/100,000; women 5.5/100,000).
Age structure and duration of SCI
All the studies, however, reported unanimously that the average age of SCI patients at the time of diagnosis was between 48 and 61 years, hence approximately 15–30 years below the average age of the general population [4–20].
Average latency between the onset of paralysis and developing bladder cancer in all the studies with a larger number of patients (n ≥ 10 patients) was between 21 and 34 years. The variation of the reported latency is wide, although bladder cancer in patients with fewer than 10 years of paralysis is a rare exception in all studies (Table 2).
Tumour characterisation and prognosis
The special histopathology and particularly aggressive nature of bladder tumours in SCI patients have already been described in earlier studies. The proportion of tumours that had already invaded the muscle at the time of diagnosis was higher [9, 20], as was the proportion of squamous cell carcinomas due to loss of differentiation (summary in [32] and Table 2). Our own data confirm this, with 19/24 invasive tumours and a proportion of SCC of 4/24.
The poor prognosis for the patients presented here, with a median survival of only 11.5 months, also corresponds to the numbers given in the literature. Broecker et al. [6] reported a median survival of 13 months, Hess et al. [16] only 7.8 months. The 1-year survival rates were 56% [16], 61% [13], 70% [11], respectively, and 50% in our own patient group.
Studies on mortality/causes of death
The few available mortality data associated with cancer of the urinary bladder in SCI patients support the assumption of a significantly higher risk of bladder cancer as the cause of death for patients with SCI.
El-Masri and Fellows [7] calculated it at 20 times higher for SCI patients.
In a recent evaluation by the National SCI Statistical Centre and the National Centre for Health Statistics in the USA, data from 45,486 patients whose paralysis was caused by traumatic injury were analysed starting from 1936 (approx. 92% of the injuries were acquired after 1970) [27], corresponding to 566,532 years of follow-up. Among the 10,575 fatalities, 99 were caused by cancer of the urinary bladder. The standardised mortality rate (SMR) for all patients with SCI was 6.69 (male: 5.96, 95% CI 4.71–7.44, female: 12.21, 95% CI 7.56–18.66), which means that their risk of death from bladder cancer is higher by a factor of 6.7 (95% CI 5.4–8.1) compared with the general population. Particular risk factors were identified as more than 10 years duration of paralysis (1–9 years SMR 1.4, 95% CI 0.57–2.93; 10–19 years SMR 3.96, 95% CI 2.34–6.25; 20 years and longer SMR 17.83, 95% CI 14.00–22.39), especially for the age group 30–59 years (SMR 19.83, 95% CI 15.49–25.02). In cases of motor functionally complete injury (AIS A, B or C [27, 33]), the SCI level influenced the mortality rate: SMR was 6.03 (95% CI 2.43–12.43) in high-level tetraplegics (C1–C4). For tetraplegics with lower level of injury C5–C8, it was 14.75 (95% CI 10.09–20.82), and in paraplegics T1–S3 12.63 (95% CI 9.34–16.70). Mortality due to bladder cancer in patients with motor functionally incomplete injury (AIS D), patients with a duration of paralysis of less than 10 years or those depending on long-term-assisted breathing was not significantly higher.
A study in England and Wales [34] of 207 women with spinal cord injury showed the odds ratio of death from bladder cancer at 12.0 (95% CI 1.5–99.7).
Groah et al. [14] calculated the bladder cancer mortality risk (SMR) per 100.000 person years, standardised by age and gender of the general US population at 70.6 (95% CI 36.9–123.3).
Indwelling catheters and urinary bladder cancer
It has been suggested that chronic indwelling transurethral and suprapubic catheters are a risk factor for developing bladder cancer [14]. According to a recent meta-analysis [35], 1% of all SCI patients with long-term indwelling catheters later developed a carcinoma of the urinary bladder. However, more than half of the reported carcinomas in those with SCI did not have an indwelling catheter. Therefore, it appears that there are also other factors responsible for the increase in bladder cancer in SCI patients.
In many studies, especially from the USA, more than half the cancer patients had an indwelling catheter (Stonehill et al. [11] 88%, West et al. [13] 62%, Groah et al. [14] 71%, Kalisvaart et al. [20] 64%, Nahm et al. [27] 42.9%).
Groah et al. [14] calculated a relative risk (RR) for patients using indwelling catheters compared to bladder management without indwelling catheters of 4.9 (95% CI 1.3–13.8, p < 0.02), Stonehill et al. [11] of 12.8. Bladder cancer incidence rose after 10–19 years with an indwelling catheter to 86.8 per 100,000 person years, and to 398 per 100,000 person years after more than 20 years (relative risk 4.6, 95% CI 1.5–14.0) [14].
In a study from the German-speaking area (Pannek [15]: 7/37 = 19%), the proportion of patients with indwelling catheters was much lower. In the present study, long-term catheterisation was virtually negligible. Thus, the two German studies also point to bladder cancer risk factors other than indwelling catheters.
However, the data situation in this case is a problem, especially as many patients change their method of drainage over time.
Whereas long-term catheterization is generally deemed a risk factor for carcinogenesis, newer studies [20] as well as our own data indicate that the risk of bladder cancer is higher than normal for SCI patients with or without permanent catheters. Thus, thinking about screening or the issue of linkages should not be exclusively limited to patients with indwelling catheters.
Urinary tract infections and bladder cancer
SCI patients with consequential neurogenic bladder dysfunction are more vulnerable to urinary tract infections (UTI) than people with normal bladder function [36, 37]. UTI increases the incidence of bladder cancer by a factor of two to four [38–41]. Only Kjaer et al. [42] were not able to confirm this association. Prospective studies on this issue are lacking [43].
In Pannek’s retrospective data survey [15], 24.3% of patients had more than 10 UTI episodes per year, and another 40.6% had chronic infection. In our own study population, 10 out of 24 patients (41.66%) reported a history of recurrent UTI (3 or more episodes per year).
Nevertheless, the overall data situation is insufficient, and the inadequacy is aggravated by the problem of definition and evaluation of asymptomatic bacteriuria, which is common in SCI patients.
Bladder stones and bladder cancer
Spinal cord injury patients often suffer from bladder stones, depending on the type of drainage [44]. The hazard ratio with a suprapubic catheter compared to intermittent catheterization was 10.5 (p < 0.0005, 95% CI 4.0–27.5) and with transurethral catheter 12.8 (p < 0.0005, 95% CI 5.1–31.9) [45]. In contrast to kidney stones, bladder stones pose an elevated risk of bladder cancer (relative risk, RR 1.8; 95% CI 1.1–2.8) [39].
Significantly higher rates of bladder stones have also been reported in SCI patients with bladder cancer [11]. Groah et al. [14] using a multivariate regression model found no significant increase in bladder stones. In our study, 5 out of 24 patients (20.8%) had a history of bladder stones.
At present, it is not possible to say whether this is an independent risk factor. However, the data indicate at least that classic “risk factors” like recurrent UTI or bladder stones are not an essential pre-condition for the development of carcinoma of the urinary bladder.
Other risk factors
Information about smoking, which is by far the most important risk factor for bladder cancer, varies within wide limits in the retrospective studies. The proportion of smokers was between 32 and 70% [15, 16, 20]. Non-smokers had a significantly better prognosis in a comparative study of SCI bladder cancer survivors and non-survivors [46].
With regard to other possible risk exposure (occupational exposure at the workplace, cyclophosphamide, radiation to the pelvis, schistosomiasis), there are no studies reporting specifically on SCI patients.
Type of lower urinary tract dysfunction
Studies on a possible correlation between the type of lower urinary tract dysfunction and bladder tumours are also absent. In the Hamburg patient collective, 3 out of 24 patients had a lesion of the lower motor neuron with acontractile detrusor.
A summary of the risk factors is given in Table 3.
Prevention
Although there are no defined specific preventive measures, Frankel et al. [47] identified a 41% difference in the risk of dying of urological complications in two different hospitals using the identical method of analysis. This underscores the necessity for managing SCI patients in highly specialised centres, and the importance of standardised neuro-urological follow-up care [48].
Screening
Even neuro-urological specialists with many years of experience in the management and care of patients with SCI are not able at present to give substantiated recommendations for a meaningful screening programme for bladder cancer in these patients.
Cameron et al. [49] concluded after a systematic review that no method of bladder cancer screening has yet been defined for SCI patients. At present, no evidence base exists for the use of urine tumour markers, cytology or annual cystoscopy with biopsies [50–53]. Sammer et al. [54] concluded from a recent study on 129 patients with neurogenic lower urinary tract dysfunction (85 with SCI) that “surveillance urethro-cystoscopy might be warranted, although the ideal starting point and frequency remain to be determined in further prospective studies”.
In this context, it should be mentioned that cystoscopy in a spinal cord lesion above T6 can trigger autonomic dysreflexia, which may result in complications ranging from hypertensive crisis to brain haemorrhage or life-threatening bradycardia [55]. Therefore, in those with injuries at T6 and above, cystoscopy needs to be done under anaesthesia or by a urology team that is very experienced in the evaluation and management of autonomic dysreflexia.
The recommendation expressed in various guidelines [56–60], that screening for bladder cancer should be performed in permanently catheterised patients after 10 years irrespective of the presence of neurogenic bladder dysfunction, is probably not adequate for patients with spinal cord injury in view of the most recent data. Any considerations in relation to screening for these patients must be based on the “years since injury”, without regard to the presence of classic risk factors such as the UTI rate or history of bladder stones, and irrespective of the type of neurogenic lower urinary tract dysfunction or bladder management.
The current EAU Neuro-Urology Guidelines [60] do not make any recommendation about screening.
In a decision analysis model for cost-effectiveness, generated for surveillance cystoscopy and cytology after augmentation cystoplasty in children with spina bifida, Eliott stated that the risk of bladder cancer in a general SCI population is not high enough to warrant screening. However, “this finding should not stop us from working to identify risk factors that place certain SCI subpopulations at a sufficiently high risk that they would warrant screening” [61]. In the view of the authors of this paper, medical benefit should weigh higher than cost-effectiveness.
Treatment standards
The same treatment guidelines as for patients without SCI apply basically to both superficial and invasive tumours. Radical cystectomy is the treatment of choice, also for SCI patients with muscle invasion. The issue of urine drainage after radical cystectomy has to be addressed to a high degree with case-by-case decisions, including the specific paralysis situation of each patient and possible functional deficits that can no longer be compensated for after radical cystectomy.
A very critical approach should be adopted with regard to the possibility of orthotopic bladder replacement, which is an option suitable only for a few individual cases. The same applies to creating a pouch with a continent stoma that can be catheterised. Only a small number of isolated case reports on this kind of procedure are in existence at present [62].
In most cases, an ileum conduit for urine drainage will probably be the most recommendable solution in view of quality of life [63].
Conclusions
Data from all observations agree that the age of SCI patients who develop the disease is well below that of the normal population indicating that bladder cancer is causatively associated with SCI.
The proportion of invasive tumours and squamous loss of differentiation is higher. Accordingly, the prognosis is much poorer than for the general population.
Available data indicate that the years since injury (hence, the duration of neurogenic bladder dysfunction) could be a crucial risk factor.
Long-term permanent catheterization must definitely be deemed a risk factor for carcinogenesis, although more recent studies and our own data show that the type of bladder drainage does not seem to be the crucial factor in association with SCI, nor does the level of injury or the form of neurogenic bladder dysfunction (neurogenic detrusor overactivity or acontractile detrusor) appear to stratify the risk according to the Hamburg data.
These data indicate that bladder cancer is a sequela of spinal cord injury if the latency is more than 10 years although the pathomechanism remains unclear. Thus, prospective multi-centre studies are urgently needed to elucidate the underlying pathomechanism.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. http://globocan.iarc.fr/Default.aspx. Accessed 16 Oct 2016
Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC (2011) Association between smoking and risk of bladder cancer among men and women. JAMA 306:737–745
Rushton L, Hutchings SJ, Fortunato L, Young C, Evans GS, Brown T et al (2012) Occupational cancer burden in Great Britain. Br J Cancer 107(Suppl 1):S3–S7
Melzak J (1966) The incidence of bladder cancer in paraplegia. Paraplegia 4:85–96
Kaufman JM, Fam B, Jacobs SC, Gabilondo F, Yalla S, Kane JP et al (1977) Bladder cancer and squamous metaplasia in spinal cord injury patients. J Urol 118:967–971
Broecker BH, Klein FA, Hackler RH (1981) Cancer of the bladder in spinal cord injury patients. J Urol 125:196–197
El-Masri WS, Fellows G (1981) Bladder cancer after spinal cord injury. Paraplegia 19:265–270
Bejany DE, Lockhart JL, Rhamy RK (1987) Malignant vesical tumors following spinal cord injury. J Urol 138:1390–1392
Bickel A, Culkin DJ, Wheeler JS (1991) Bladder cancer in spinal cord injury patients. J Urol 146:1240–1242
Chao R, Clowers D, Mayo ME (1993) Fate of upper urinary tracts in patients with indwelling catheters after spinal cord injury. Urology 42:259–262
Stonehill WH, Dmochowski RR, Patterson AL, Cox CE (1996) Risk factors for bladder tumors in spinal cord injury patients. J Urol 155:1248–1250
Vereczkey ZA, Schmeidler J, Binard JE, Bauman WA (1998) Bladder cancer risk in patients with spinal cord injury. J Spinal Cord Med 21:230–239
West DA, Cummings JM, Longo WE, Virgo KS, Johnson FE, Parra RO (1999) Role of chronic catheterization in the development of bladder cancer in patients with spinal cord injury. Urology 53:292–297
Groah SL, Weitzenkamp DA, Lammertse DP, Whiteneck GG, Lezotte DC, Hamman RF (2002) Excess risk of bladder cancer in spinal cord injury: evidence for an association between indwelling catheter use and bladder cancer. Arch Phys Med Rehab 83:346–351
Pannek J (2002) Transitional cell carcinoma in patients with spinal cord injury: A high risk malignancy? Urology 59:240–244
Hess MJ, Zhan EH, Foo DK, Yalla SV (2003) Bladder cancer in patients with spinal cord injury. J Spinal Cord Med 26:335–338
Subramonian K, Cartwright RA, Harnden P, Harrison SC (2004) Bladder cancer in patients with spinal cord injuries. BJU Int 93:739–743
Parra J, Drouin S, Comperat E, Misraï V, Van Glabeke E, Richard F, Denys P et al (2007) Bladder cancer in neurological patients: analysis of a single-centre series. Prog Urol 17:1333–1336 (French)
Cheng JN, Lawrentschuk N, Gyomber D, Rogerson J, Bolton DM (2010) Cystectomy in patients with spinal cord injury: indications and long-term outcomes. J Urol 184:92–98
Kalisvaart JF, Katsumi HK, Ronningen LD, Hovey RM (2010) Bladder cancer in spinal cord injury patients. Spinal Cord 48:257–261
Thietje R, Pouw MH, Schulz AP, Kienast B, Hirschfeld S (2011) Mortality in patients with traumatic spinal cord injury: descriptive analysis of 62 deceased subjects. J Spinal Cord Med 34:482–487
National Spinal Cord Injury Statistical Center: Spinal Cord Injury (SCI) Facts and figures at a glance. University of Alabama at Birmingham, Birmingham, February 2014. https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202014.pdf. Accessed 16 Oct 2016
Robert Koch Institute (ed.) and the Association of Population-based Cancer Registries in Germany (ed.). Cancer in Germany 2009/2010. 9th edition. Berlin, 2014. http://www.krebsdaten.de/Krebs/EN/Content/Publications/Cancer_in_Germany/cancer_chapters_2009_2010/cancer_germany_2009_2010.pdf?__blob=publicationFile. Accessed 16 Oct 2016
Johnson DE, Schoenwald MB, Ayala AG, Miller LS (1976) Squamous cell carcinoma of the bladder. J Urol 115:542–544
Quilty PM, Duncan W (1986) Radiotherapy for squamous carcinoma of the urinary bladder. Int J Radiat Oncol Biol Phys 12:861–865
vom Dorp F, Eisenhardt A, Goebell PJ, Gschwend J, Jäger T, Jakse G et al (2016) Harnblasenkarzinom. http://www.forschungsverbund-blasenkarzinom.de/hintergrund_hbk.pdf. Accessed 16 Oct 2016 (German)
Nahm LS, Chen Y, DeVivo MJ, Lloyd LK (2015) Bladder cancer mortality after spinal cord injury over 4 decades. J Urol 193:1923–1928
Robert Koch Institute (ed.) and the Association of Population-based Cancer Registries in Germany (ed.). Cancer in Germany 2005/2006. 7th edition. Berlin, 2010 http://www.ekr.med.uni-erlangen.de/GEKID/Doc/kid2010_english.pdf. Accessed 19 Dec 2015
Lee WY, Sun LM, Lin CL, Liang JA, Chang YJ, Sung FC et al (2014) Risk of prostate and bladder cancers in patients with spinal cord injury: a population-based cohort study. Urol Oncol 32(51):e1–e7
Ho CH, Sung KC, Lim SW, Liao CH, Liang FW, Wang JJ, Wu CC (2015) Chronic indwelling urinary catheter increase the risk of bladder cancer, even in patients without spinal cord injury. Medicine (Baltimore) 94:e1736
Zentrum für Krebsregisterdaten. Robert-Koch-Institut. http://www.krebsdaten.de/Krebs/SiteGlobals/Forms/Datenbankabfrage/datenbankabfrage_stufe2_form.html. Accessed 16 Oct 2016. (German)
Welk B, McIntyre A, Teasell R, Potter P, Loh E (2013) Bladder cancer in individuals with spinal cord injuries. Spinal Cord 51:516–521
American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury. http://asia-spinalinjury.org/wp-content/uploads/2016/02/International_Stds_Diagram_Worksheet.pdf. Accessed 16 Oct 2016
Dolin PJ, Darby SC, Beral V (1994) Paraplegia and squamous cell carcinoma of the bladder in young women: findings from a case-control study. Br J Cancer 70:167–168
Hollingsworth JM, Rogers MA, Krein SL, Hickner A, Kuhn L, Cheng A et al (2013) Determining the noninfectious complications of indwelling urethral catheters: a systematic review and meta-analysis. Ann Intern Med 159:401–410
Rabadi MH, Aston C (2015) Complications and urologic risks of neurogenic bladder in veterans with traumatic spinal cord injury. Spinal Cord 53:200–203
Siroky MB (2002) Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 113(Suppl. 1A):67S–79S
Kälble T (2001) Etiopathology, risk factors, environmental influences and epidemiology of bladder cancer. Urol A 40:447–450 (German)
Kantor AF, Hartge P, Hoover RN, Narayana AS, Sullivan JW, Fraumeni JF Jr (1984) Urinary tract infection and risk of bladder cancer. Am J Epidemiol 119:510–515
Kunze E, Chang-Claude J, Frentzel-Beyme R (1992) Life style and occupational risk factors for bladder cancer in Germany. Cancer 69:1776–1790
Vermeulen SH, Hanum N, Grotenhuis AJ, Castaño-Vinyals G, van der Heijden AG, Aben KK et al (2015) Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. Br J Cancer 112:594–600
Kjaer SK, Knudsen JB, Sørensen BL, Møller Jensen O (1989) The Copenhagen case-control study of bladder cancer. V. Review of the role of urinary-tract infection. Acta Oncol 28:631–636
Abol-Enein H (2008) Infection: Is it a cause of bladder cancer? Scand J Urol Nephrol Suppl 218:79–84
Bartel P, Krebs J, Wöllner J, Göcking K, Pannek J (2014) Bladder stones in patients with spinal cord injury: a long-term study. Spinal Cord 52:295–297
Ord J, Lunn D, Reynard J (2003) Bladder management and risk of bladder stone formation in spinal cord injured patients. J Urol 170:1734–1737
Groah SL, Lammertse DP (2003) Factors associated with survival after bladder cancer in spinal cord injury. Spinal Cord Med 26:339–344
Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA et al (1998) Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord 36:266–274
Domurath B, Kutzenberger J (2012) Modern neurological treatment strategies for patients with spinal cord injury. Urol A 51:184–188 (German)
Cameron AP, Rodriguez GM, Schomer KG (2012) Systematic review of urological followup after spinal cord injury. J Urol 187:391–397
Davies B, Chen JJ, McMurry T, Landsittel D, Lewis N, Brenes G et al (2005) Efficacy of BTA stat, cytology, and survivin in bladder cancer surveillance over 5 years in patients with spinal cord injury. Urology 66:908–911
Konety BR, Nguyen TS, Brenes G, Sholder A, Lewis N, Bastacky S et al (2000) Clinical usefulness of the novel marker BLCA-4 for the detection of bladder cancer. J Urol 164(3 Pt 1):634–639
Yang CC, Clowers DE (1999) Screening cystoscopy in chronically catheterized spinal cord injury patients. Spinal Cord 37:204–207
Hamid R, Bycroft J, Arya M, Shah PJ (2003) Screening cystoscopy and biopsy in patients with neuropathic bladder and chronic suprapubic indwelling catheters: Is it valid? J Urol 170(2 Pt 1):425–427
Sammer U, Walter M, Knüpfer SC, Mehnert U, Bode-Lesniewska B, Kessler TM (2015) Do we need surveillance urethro-cystoscopy in patients with neurogenic lower urinary tract dysfunction? PLoS ONE 10(10):e0140970
Cragg J, Krassioukov A (2012) Autonomic dysreflexia. CMAJ 184:66
Paralyzed Veterans of America. Bladder management for adults with spinal cord injury: a clinical practice guideline. http://www.pva.org/site/apps/ka/ec/product.asp?c=ajIRK9NJLcJ2E&b=6423003&en=ajIJKXODK9ISJ7NGK8KPI3MQJnLUI3OIJhLVLePYIxF&ProductID=883860. Accessed 16 Oct 2016
DMGP. Manual zur neuro-urologischen Diagnostik und Therapie Querschnittgelähmter. http://www.dmgp.de/index.php/neuro-urologie. Accessed 16 Oct 2016. (German)
SCIRE. Bladder management following spinal cord injury. http://www.scireproject.com/sites/default/files/bladder_management.pdf. Accessed 16 Oct 2016
EAU. Guidelines on urological infections. http://uroweb.org/wp-content/uploads/EAU-Guidelines-Urological-Infections-v2.pdf. Accessed 16 Oct 2016
EAU. Guidelines on neuro-urology. http://uroweb.org/wp-content/uploads/EAU-Guidelines-Neuro-Urology-2015-v2.pdf. Accessed 16 Oct 2016
Elliott SP (2015) Screening for bladder cancer in individuals with spinal cord injury. J Urol 193:1880–1881
Maurice MJ, Meeks JJ, Smith ND (2008) Orthotopic neobladder for bladder cancer and neurogenic bladder dysfunction. Can J Urol 15:4194–4195
Guillotreau J, Castel-Lacanal E, Roumiguié M, Bordier B, Doumerc N, De Boissezon X et al (2011) Prospective study of the impact on quality of life of cystectomy with ileal conduit urinary diversion for neurogenic bladder dysfunction. Neurourol Urodyn 30:1503–1506
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The institutional review board considers that this study (retrospective study) does not require informed consent.
Rights and permissions
About this article
Cite this article
Böthig, R., Kurze, I., Fiebag, K. et al. Clinical characteristics of bladder cancer in patients with spinal cord injury: the experience from a single centre. Int Urol Nephrol 49, 983–994 (2017). https://doi.org/10.1007/s11255-017-1570-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1570-6