Abstract
Purpose
Mirabegron, a potent and selective β3-adrenoceptor agonist, has been developed for the treatment of overactive bladder (OAB). We carried out a systematic review and meta-analysis to assess the efficacy and safety of the drug for treating OAB.
Methods
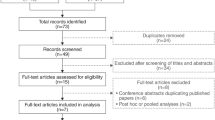
A literature review was performed to identify all published randomized double-blind, placebo-controlled phase III trials of mirabegron for the treatment of OAB. The search included the following databases: MEDLINE, EMBASE and the Cochrane Controlled Trials Register. The reference lists of the retrieved studies were also investigated. A systematic review and meta-analysis of phase III trials were conducted.
Results
Four publications involving a total of 5,761 patients were used in the analysis, including four phase III RCTs that compared mirabegron with placebo. We found that mirabegron was effective in treating OAB in our meta-analysis. Co-primary efficacy end points: the mean number of incontinence episodes per 24 h (the standardized mean difference (SMD) = −0.44, 95 % confidence interval (CI) −0.59 to −0.29, p < 0.00001); the mean number of micturitions per 24 h (SMD = −0.62, 95 % CI −0.80 to −0.45, p < 0.00001) and key secondary efficacy end points: mean volume voided per micturition; mean number of urgency episodes per 24 h indicated that mirabegron was more effective than the placebo. Safety assessments included common treatment-emergent adverse events (TEAEs) [OR 1.10, 95 % CI 0.93–1.31, p = 0.25), hypertension, cardiac arrhythmia TEAEs, urinary retention and discontinuations due to adverse event indicated that mirabegron was well tolerated.
Conclusions
This meta-analysis indicates that mirabegron to be an effective and safe treatment for OAB symptoms with a low occurrence of side effects. It offers promise as an effective oral agent for the treatment of OAB with a distinct efficacy/tolerability balance.





Similar content being viewed by others
References
Abrams P, Cardozo L, Fall M et al (2002) The standardisation of terminology of lower urinary tract function: report from the standardisation subcommittee of the international continence society. Neurourol Urodyn 21:167–178
Irwin D, Milsom I, Hunskaar S et al (2006) Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 50:1306–1314
Sacco E, Tienforti D, D’Addessi A et al (2010) Social, economic, and health utility considerations in the treatment of overactive bladder. J Urol 2:11–24
Chapple CR (2000) Muscarinic receptor antagonists in the treatment of overactive bladder. Urology 55:33
Garely AD, Borrows LJ (2002) Current pharmacotherapeutic strategies for overactive bladder. Expert Opin Pharmacother 3:827
Abrams P, Andersson KE, Buccafusco JJ et al (2006) Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol 148:565–578
D’Souza AO, Smith MJ, Miller LA et al (2008) Persistence, adherence, and switch rates among extended-release and immedia terelease overactive bladder medications in a regional managed care plan. J Manag Care Pharm 14:291–301
Benner JS, Nichol MB, Rovner ES et al (2010) Patient-reported reasons for discontinuing overactive bladder medication. BJU Int 105:1276–1282
Takasu T, Ukai M, Sato S et al (2007) Effect of (R)-2-(2-aminothiazol-4-yl)-4′-{2-[(2-hydroxy-2-phenyl-ethyl)amino]ethyl} acetanilide (YM178), a novel selective beta3-adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther 321:642
Takeda M, Obara K, Mizusawa T et al (1999) Evidence for beta3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther 288:1367–1373
Igawa Y, Aizawa N, Homma Y (2010) Beta3-adrenoceptor agonists: possible role in the treatment of overactive bladder. Korean J Urol 51:811–818
Andersson K, Chapple C, Cardozo L et al (2009) Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Khoury S, Wein A (eds) Incontinence, 4th international consultation on incontinence. Plymbridge Distributors, Plymouth, pp 631–699
Andersson K, Arner A (2004) Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84:935–986
Otsuka A, Shinbo H, Matsumoto R et al (2008) Expression and functional role of beta-adrenoceptors in the human urinary bladder urothelium. Naunyn Schmiedebergs Arch Pharmacol 377:473–481
Limberg B, Andersson K, Aura KF et al (2010) β-Adrenergic receptor subtype expression in myocyte and non-myocyte cells in human female bladder. Cell Tissue Res 342:295–306
Jadad AR (1998) Randomised controlled trials. BMJ, London
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions, v.5.1. Cochrane collaboration web site. http://www.cochrane-handbook.org/
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Khullar V, Amarenco G, Angulo JC et al (2013) Efficacy and tolerability of mirabegron, a β (3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European–Australian phase 3 trial. Eur Urol 63(2):283
Nitti VW, Auerbach S, Martin N et al (2013) Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 189:1388
Van KP, Barkin J, Castro-Diaz D et al (2013) Randomised, double-blind, placebo-controlled phase III study to assess the efficacy and safety of mirabegron 25 mg and 50 mg once daily in overactive bladder (OAB). In: Presented at the international continence society meeting, Beijing, China, 2012. Available at http://www.icsoffice.org/Abstracts/Publish/134/000359.pdf. Accessed 17 Feb 2013
Edwards SJ, Karner C, Trevor N et al (2013) Mirabegron for the treatment of symptoms associated with overactive bladder. BMJ-TAG, London
Chapple CR, Kaplan SA, Mitcheson D et al (2013) Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a β(3)-adrenoceptor agonist, in overactive bladder. Eur Urol 63(2):296
Wagg A, Compion G, Fahey A et al (2012) Persistence with prescribed antimuscarinic therapy for overactive bladder: a UK experience. BJU Int. doi:10.1111/j.1464-410X.2012.11023.x
Linner L, Schioler H, Samuelsson E et al (2011) Low persistence of anticholinergic drug use in Sweden. Eur J Clin Pharmacol 67:535–536
Yamanishi T, Chapple CR, Yasuda K et al (2003) Role of beta-adrenoceptor subtypes in mediating relaxation of the pig bladder trigonal muscle in vitro. Neurourol Urodyn 22:338
Conflict of interest
The authors had no conflicts of interest to declare in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Huantao Zong is the co-first author.
Rights and permissions
About this article
Cite this article
Cui, Y., Zong, H., Yang, C. et al. The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. Int Urol Nephrol 46, 275–284 (2014). https://doi.org/10.1007/s11255-013-0509-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-013-0509-9