Abstract
A common and potent consideration has recently entered the landscape of the novel coronavirus disease of 2019 (COVID-19): venous thromboembolism (VTE). COVID-19 has been associated to a distinctive related coagulopathy that shows unique characteristics. The research community has risen to the challenges posed by this « evolving COVID-19 coagulopathy » and has made unprecedented efforts to promptly address its distinct characteristics. In such difficult time, both national and international societies of thrombosis and hemostasis released prompt and timely responses to guide recognition and management of COVID-19-related coagulopathy. However, latest guidelines released by the international Society on Thrombosis and Haemostasis (ISTH) on May 27, 2020, followed the American College of Chest Physicians (CHEST) on June 2, 2020 showed some discrepancies regarding thromboprophylaxis use. In this forum article, we would like to offer an updated focus on thromboprophylaxis with current incidence of VTE in ICU and non-ICU patients according to recent published studies; highlight the main differences regarding ISTH and CHEST guidelines; summarize and describe which are the key ongoing RCTs testing different anticoagulation strategies in patients with COVID-19; and finally set a proposal for COVID-19 coagulopathy specific risk factors and dedicated trials.
Similar content being viewed by others
-
Reported incidence of venous thrombotic events in COVID-19 patients
-
Major differences between ISTH and CHEST guidelines in thromboprophylaxis for patients with COVID-19
-
Ongoing RCTs of different anticoagulation strategies in patients with COVID-19
-
A proposal for COVID-19 coagulopathy specific risk factors and dedicated trials
A common and potent consideration has recently entered the landscape of the novel coronavirus disease of 2019 (COVID-19): venous thromboembolism (VTE). COVID-19 has been associated to a distinctive related coagulopathy that shows unique characteristics [1]. The research community has risen to the challenges posed by this « evolving COVID-19 coagulopathy » and has made unprecedented efforts to promptly address its distinct characteristics. However, a key central question that could guide prevention, diagnosis, and treatment strategies of COVID-19 coagulopathy remains under debate: are these haemostatic changes a consequence of severe inflammation or are they a specific effect mediated by the virus? [2]. The immune response to acute SARS-CoV-2 infection and the accompanying surge of cytokines and inflammatory mediators have been accepted as a key pathway triggering thrombogenesis. In this setting, early strategies aimed at reducing inflammation might help prevent thrombosis. The alternative postulate is that the virus directly or indirectly interferes with coagulation pathways. The determinants of both hypotheses seem to stem mostly from host factors such as age, comorbidities, and the prominent role played by the extent of lung injury. Owing to these determinants, the combined use of risk scores to identify high-risk patients for adverse thrombotic events may guide individualized antithrombotic treatment of Covid-19 patients [3]. Another important insight is the recognition of the importance of extravascular fibrinolytic activity in the airway lumen and the alveolar compartment. Extravascular fibrin was demonstrated as a possible mechanism by which inflammatory cells can invade the lung [4]. Breakdown of fibrin as a consequence of high fibrinolytic activity would lead to a marked generation of D-dimers levels independently of thrombotic events. According to this paradigm, high D-dimers levels would not be solely considered as a marker of thrombotic propensity but should be viewed as an integrate marker of disease severity including the extent of lung damage [5].
In the inpatient setting, the prevalence of VTE ranges from 3 to 85%, as detailed in Fig. 1 [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25].
However, most of studies on coronavirus patients used different design (systematic screening vs D-Dimer threshold vs symptom-driven approach), different intervention (contrasting intensities of thromboprophylaxis regimens), severity (ICU vs wards) and outcome (asymptomatic vs symptomatic VTE) resulting in reduced data comparability across studies (Table 1).
Furthermore, investigations from the outpatients are warranted with high priority, as they represent the vast majority of Covid-19 cases and VTE rate in this specific subset has not been reported yet [26]. Early reports suggested a high incidence of VTE and frequent haemostasis disorders in COVID-19 patients [27, 28]. Though, it remains to be demonstrated that theses frequent «new thrombotic» features at first glance are any different from previous experience from severe viral pneumonia [29,30,31,32,33]. Both intrinsic and extrinsic risk factors for VTE (Fig. 2) together with large number of patients considered at high risk on the basis of current VTE risk scores [34] lead to first interim [35] followed by updated guidance on thromboprophylaxis in hospitalized patients with COVID-19 [36, 37].The first reminder of a beneficial effect of thromboprophylaxis came as early as March 27, 2020 with reduced mortality in critically ills affected by severe COVID-19 and treated with heparin [38]. Of note, only 22.0% of the population analyzed by Tang et al. received anticoagulant therapy for the prevention of VTE and this reinforced the role for routine VTE risk assessment and the initiation of adequate thromboprophylaxis [39]. A substantial 5 to 10% risk of VTE in critically ills is currently reported despite the use of prophylactic anticoagulants [40,41,42,43]. COVID-19 patients presented in later reports with unusual higher rates of VTE despite the use of prophylactic anticoagulants [6,7,8,9, 12, 21].
Latest ISTH consensus statement published on May 27, 2020 recommended routine thromboprophylaxis in non-ICU and ICU hospitalized COVID-19 patients with preferably standard-dose LMWH or UFH [37]. Due to time-sensitivity with the pandemic and in the absence of robust evidence, a “stepped therapy” approach in non-ICU patients or treatment-dose heparin in critically ills did not reach full consensus yet. With regards to the rapid deterioration reported in many COVID-19 patients requiring ICU transfer, long half-life and/or reversibility concerns, both fondaparinux and prophylactic dose DOAC were not recommended in critically ill hospitalized COVID-19 patients. Apart from body weight-adjusted dose on extremes cases (< 50 kg or > 120 kg or BMI), the ISTH expert panel recommended against the general use of intermediate dose of LMWH/UFH in non-ICU. Wisely awaiting for some strong evidences, intermediate-dose LMWH was only advocated by 30% of ISTH respondent in non-ICU and up to 50% in ICU patients (Table 2).
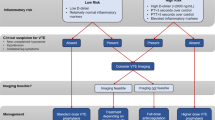
No more that 6 days after the ISTH guidance had been released, an American College of Chest Physicians (CHEST) panel of experts provided a conflicting set of guidelines on June 2, 2020 [44]. CHEST experts recommended (i) standard dose anticoagulant thromboprophylaxis in non-ICU and ICU patients, (ii) LMWH or fondaparinux over UFH in non-ICU patients, (iii) suggested against the addition of mechanical prophylaxis (i.e. intermittent pneumatic compression) to pharmacological thromboprophylaxis while 60% of ISTH experts pledged for it. Armed with this two set of guidelines, one being « conservative » and the other much more « liberal» on both stepped-up pharmacological and mechanical approach, how is the physician supposed to react in day use practice? Both guidelines nonetheless advocated for more evidence coming from ongoing randomized trials (Table 3), more extensive description of the « sicker » or « higher risk » patient profile likely to benefit from increased intensity anticoagulant thromboprophylaxis, and finally a call for updated evidences regarding bleeding risk in this population as they are insufficient so far. Identifying very-high-risk patients for VTE is undoubtedly the main issue of reducing both incidence and mortality risk of VTE [45]. The triad of risk seems to essentially rely on marked prothrombotic state, thromboinflammation and the extent of lung injury (Fig. 3).
All studies of haemostasis have identified a prothrombotic state in COVID-19 [46]. Thachil et al. lately proposed a new staging classification characterizing COVID-19 associated hemostatic abnormalities (CAHA) [3]. The authors proposed that the spectrum of CAHA first represents a localized phenomenon of hypercoagulability in the lung, which then becomes extensive and systemic (increased D-Dimer level, reduced platelet count and prolonged PT) if not treated adequately. We promptly confirmed a stepwise increase in VTE rates and excess mortality and/or transfer to ICU for each increment in stage of CAHA among 150 non-ICU patients with COVID-19 [47]. Hence, we proposed a CAHA threshold ≥ 2 to consider early aggressive strategies including early VTE imaging screening, “stepped-up” anticoagulant dose regimens and critical care support. VTE risk stratification scheme and prospective RCTs are needed to determine whether intermediate or treatment-dose anticoagulant confer both survival benefit and decreased VTE incidence according to biomarkers threshold including the use of very elevated D-dimer levels and inflammatory markers in hospitalized patients with COVID-19.
Hyperinflammation has been advocated as a key component triggering thromboinflammation and subsequent increased risk of VTE [48, 49]. The first event after inhalation of SARS coronaviruses is invasion of type II alveolar cells in the lung. Viral cell entry triggers the host’s immune response and an inflammatory cascade. While viral multiplication and localized inflammation in the lung is the norm, severe COVID-19 patients will develop an overproduction of proinflammatory cytokines resulting in a cytokine storm [50]. On top of anti-inflammatory or antiviral effects, current therapeutic strategies (e.g. intravenous immunoglobulin, selective cytokine blockade etc.) [51] may have indirect antithrombotic effects and modulate the risk of VTE.
Lung and pulmonary thrombosis have an intimate relationship in COVID-19. The first hint came from accumulating evidence of published necropsy series with the prominence of clot, widespread micro-thrombi and occlusion of alveolar capillaries [26, 52,53,54]. More evidence followed with proof of pulmonary endotheliitis in the time course of SARS-CoV-2 infection [55]. A distinctive pattern of pulmonary intravascular coagulopathy has finally been proposed [56, 57]. The current consensus puts the lungs as the epicenter for the hemostatic and inflammatory issues in COVID-19. Desborough et al. nicely addressed this issue providing evidence that many of the acute pulmonary embolism are indeed described on CT pulmonary angiograms as segmental or subsegmental and that these thromboses may be immunothromboses due to local inflammation, rather than thromboembolic disease [58]. First localized to the lung, then extensive and finally systemic if not treated, the phenomenon of pulmonary intravascular coagulopathy in COVID-19 pneumonia translates in clinical practice with higher oxygen requirement and extensive lung injuries assessed by chest CT [18, 47, 59].
Several anticoagulant regimens are been currently investigated in patients with COVID-19. Systematic screening for marked prothrombotic state, hyperinflammation and the extent of lung injury as determined by chest CT could be helpful to guide individualized thromboprophylaxis in COVID-19 patients.
Abbreviations
- CA:
-
Chronic therapeutic anticoagulation
- BID:
-
Twice-daily
- BMI:
-
Body mass index
- COVID-19:
-
Coronavirus disease 2019
- CT:
-
Computed tomography
- DOAC:
-
Direct oral anticoagulant
- DVT:
-
Deep vein thrombosis
- ICU:
-
Intensive care unit
- IT:
-
Thromboprophylaxis with intermediate dose of LMWH/ UFH
- LMWH:
-
Low molecular weight heparin
- N/A:
-
Not available
- PE:
-
Pulmonary embolism
- RCTs:
-
Randomized controlled trials
- SD:
-
Routine thromboprophylaxis with standard dose of UFH or LMWH
- TD:
-
Thromboprophylaxis with therapeutic dose
- UFH:
-
Unfractionated heparin
- VTE:
-
Venous thromboembolism
References
Marchandot B, Sattler L, Jesel L, Matsushita K, Schini-Kerth V, Grunebaum L, Morel O (2020) COVID-19 related coagulopathy: a distinct entity? A review. J Clin Med 9:1651
The Lancet Haematology (2020) COVID-19 coagulopathy: an evolving story. Lancet Haematol. https://doi.org/10.1016/S2352-3026(20)30151-4
Thachil J, Cushman M, Srivastava A (2020) A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. https://doi.org/10.1002/rth2.12372
Wagers SS, Norton RJ, Rinaldi LM, Bates JH, Sobel BE, Irvin CG (2004) Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 114(1):104–111. https://doi.org/10.1172/JCI19569
Thachil J (2020) All those D-dimers in COVID-19. J Thromb Haemost. https://doi.org/10.1111/jth.14939.10.1111/jth.14939
Klok FA, Kruip M, van der Meer N, Arbous MS, Gommers D, Kant KM, Kaptein F, van Paassen J, Stals M, Huisman MV, Endeman H (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147. https://doi.org/10.1016/j.thromres.2020.04.013
Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F, CRICS TRIGGERSEP Group (Clinical Research in Intensive Care, and Sepsis Trial Group for Global Evaluation, and Research in Sepsis) (2020) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens Care Med 46(6):1089–1098. https://doi.org/10.1007/s00134-020-06062-x
Maatman TK, Jalali F, Feizpour C, Douglas A 2nd, McGuire SP, Kinnaman G, Hartwell JL, Maatman BT, Kreutz RP, Kapoor R, Rahman O, Zyromski NJ, Meagher AD (2020) Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe coronavirus disease 2019. Crit Care Med. https://doi.org/10.1097/CCM.0000000000004466
Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S, Haemostasis LICU, COVID-19 group (2020) Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.047430
Cui S, Chen S, Li X, Liu S, Wang F (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. https://doi.org/10.1111/jth.14830
Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller M, Bouman C, Beenen L, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N (2020) Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. https://doi.org/10.1111/jth.14888
Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT, Barco S, Humanitas COVID-19 Task Force (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191:9–14. https://doi.org/10.1016/j.thromres.2020.04.024
Ren B, Yan F, Deng Z, Zhang S, Xiao L, Wu M, Cai L (2020) Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.047407
Grillet F, Behr J, Calame P, Aubry S, Delabrousse E (2020) Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. https://doi.org/10.1148/radiol.2020201544
Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K (2020) High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. https://doi.org/10.1111/jth.14869
Longchamp A, Longchamp J, Manzocchi-Besson S, Whiting L, Haller C, Jeanneret S, Godio M, Martinez JJG, Bonjour T, Caillat M, Maitre G, Thaler JM, Pantet R, Donner V, Dumoulin A, Emonet S, Greub G, Friolet R, Robert-Ebadi H, Righini M, Sanchez B, Delayoe J (2020) Venous thromboembolism in critically ill patients with Covid-19: results of a screening study for deep vein thrombosis. JTH. https://doi.org/10.1002/rth2.12376
Galeano-Valle F, Oblitas CM, Ferreiro-Mazón MM, Alonso-Muñoz J, Del Toro-Cervera J, Demelo-Rodríguez P (2020) Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb Res. https://doi.org/10.1016/j.thromres.2020.05.017
Trimaille A, Curtiaud A, Marchandot B, Matsushita K, Sato C, Leonard-Lorant I, Sattler L, Grunebaum L, Ohana M, Von Hunolstein JJ, Andre E, Goichot B, Danion F, Kaeuffer C, Poindron C, Ohlmann P, Jesel L, Morel O (2020) Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thrombosis Res. https://doi.org/10.1016/j.thromres.2020.07.033
Demelo-Rodríguez P, Cervilla-Muñoz E, Ordieres-Ortega L, Parra-Virto A, Toledano-Macías M, Toledo-Samaniego N, García-García A, García-Fernández-Bravo I, Ji Z, de-Miguel-Diez J, Álvarez-Sala-Walther LA, Del-Toro-Cervera J, Galeano-Valle F (2020) Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 192:23–26. https://doi.org/10.1016/j.thromres.2020.05.018
Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, Zhang C, Li H, Xia X, Kong S, Liao J, Jia H, Pang X, Song Y, Tian Y, Wang B, Wu C, Yuan H, Zhang Y, Li Y, Sun W, Zhang Y, Zhu S, Wang S, Xie Y, Ge S, Zhang L, Hu Y, Xie M (2020) Deep vein thrombosis in hospitalized patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: prevalence, risk factors, and outcome. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.046702
Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, Néel A, Lecomte R (2020) Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis 25:1–6. https://doi.org/10.1007/s11239-020-02146-z
Voicu S, Bonnin P, Stépanian A, Chousterman BG, Le Gall A, Malissin I, Deye N, Siguret V, Mebazaa A, Mégarbane B (2020) High prevalence of deep vein thrombosis in mechanically ventilated COVID-19 patients. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2020.05.053
Nahum J, Morichau-Beauchant T, Daviaud F, Echegut P, Fichet J, Maillet JM, Thierry S (2020) Venous thrombosis among critically ill patients with coronavirus disease 2019 (COVID-19). JAMA Netw Open 3(5):e2010478. https://doi.org/10.1001/jamanetworkopen.2020.10478
Santoliquido A, Porfidia A, Nesci A, De Matteis G, Marrone G, Porceddu E, Cammà G, Giarretta I, Fantoni M, Landi F, Gasbarrini A, Pola R. Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. JTH https://doi.org/10.1111/JTH.14992
Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N et al (2020) Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa500
Benzakoun J, Hmeydia G, Delabarde T, Hamza L, Meder JF, Ludes B, Mebazaa A (2020) Excess out-of-hospital deaths during COVID-19 outbreak: evidence of pulmonary embolism as a main determinant. Eur Heart Fail. https://doi.org/10.1002/ejhf.1916
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS et al (2020) China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:844–847
Yin S, Huang M, Li D, Tang N (2020) Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis. https://doi.org/10.1007/s11239-020-02105-8
Chong PY, Chui P, Ling AE, Franks TJ, Tai DY, Leo YS, Kaw GJ, Wansaicheong G, Chan KP, Ean Oon LL, Teo ES, Tan KB, Nakajima N, Sata T, Travis WD (2004) Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med 128(2):195–204. https://doi.org/10.1043/1543-2165(2004)128<195:AODDTS>2.0.CO;2
Giannis D, Ziogas IA, Gianni P (2020) Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. https://doi.org/10.1016/j.jcv.2020.104362
Obi AT, Tignanelli CJ, Jacobs BN, Arya S, Park PK, Wakefield TW, Henke PK, Napolitano LM (2019) Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg 7(3):317–324. https://doi.org/10.1016/j.jvsv.2018.08.010
Levi M, Schultz M, van der Poll T (2010) Disseminated intravascular coagulation in infectious disease. Semin Thromb Hemost 36(4):367–377. https://doi.org/10.1055/s-0030-1254046
Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S (2020) Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haemat 7(5):e362–e363. https://doi.org/10.1016/S2352-3026(20)30109-5
Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T (2020) ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18:1023–1026
Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian CD, Ageno W, Madjid M, Guo Y et al (2020) COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol
Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD (2020) Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis, Haemostasis. Scientific and Standardization Committee Communication: Clinical Guidance on the Diagnosis, Prevention and Treatment of Venous Thromboembolism in Hospitalized Patients with COVID-19. J Thromb Haemost. https://doi.org/10.1111/jth.14929
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. https://doi.org/10.1111/jth.14817
Porfidia A, Pola R (2020) Venous thromboembolism in COVID-19 patients. J Thromb Haemost. https://doi.org/10.1111/jth.14842
PROTECT Investigators for the Canadian Critical Care Trials Group, and the Australian, and New Zealand Intensive Care Society Clinical Trials Group, Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, Crowther M, Geerts W, Cooper DJ, Vallance S, Qushmaq I, Rocha M, Berwanger O, Vlahakis NE (2011) Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med 364(14):1305–1314. https://doi.org/10.1056/NEJMoa1014475
Cade JF (1982) High risk of the critically ill for venous thromboembolism. Crit Care Med 10:448–450
Kapoor M, Kupfer YY, Tessler S (1999) Subcutaneous heparin prophylaxis significantly reduces the incidence of venous thromboembolic events in the critically ill [abstract]. Crit Care Med 27:A69
Fraisse F, Holzapfel L, Couland JM, Simonneau G, Bedock B, Feissel M, Herbecq P, Pordes R, Poussel JF, Roux L (2000) Nadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. The Association of Non-University Affiliated Intensive Care Specialist Physicians of France. Am J Respir Crit Care Med 161(4):1109–1114. https://doi.org/10.1164/ajrccm.161.4.9807025
Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Holley AB, Jimenez D, LeGal G, Rali P, Wells P (2020) Prevention, diagnosis and treatment of venous thromboembolism in patients with COVID-19: CHEST Guideline and Expert Panel Report. Chest. https://doi.org/10.1016/j.chest.2020.05.559
Thachil J, Juffermans NP, Ranucci M, Connors JM, Warkentin TE, Ortel TL, Levi M, Iba T, Levy JH (2020) ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost. https://doi.org/10.1111/JTH.15004
Polimeni A, Leo I, Spaccarotella C, Mongiardo A, Sorrentino S, Sabatino J, Rosa SD, Indolfi C (2020) Prognostic impact of coagulopathy in patients with COVID-19: a meta-analysis of 35 studies and 6427 patients. Res Sq. https://doi.org/10.21203/rs.3.rs-31142/v1
Marchandot B, Trimaille A, Curtiaud A, Carmona A, Matsushita K, Sato C, Leonard-Lorant I, Sattler L, Grunebaum L, Ohana M, Ohlmann P, Jesel L, Morel O. Staging COVID-19 coagulopathy in non-critically ill patient (in review)
Siddiqi HK, Mehra MR (2020) COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 39(5):405–407. https://doi.org/10.1016/j.healun.2020.03.012
Connors JM, Levy JH (2020) Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. https://doi.org/10.1111/jth.14849
Jose RJ, Manuel A (2020) COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30216-2
Bikdeli B, Madhavan MV, Gupta A, Jimenez D, Burton JR, Der Nigoghossian C, Chuich T, Nouri SN, Dreyfus I, Driggin E, Sethi S, Sehgal K, Chatterjee S, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Bertoletti L, Giri J, Global COVID-19 Thrombosis Collaborative Group et al (2020) Pharmacological Agents Targeting Thromboinflammation in COVID-19: Review and Implications for Future Research. Thromb Haemost. https://doi.org/10.1055/s-0040-1713152
Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, Ferraz Da Silva LF, Pierre de Oliveira E, Nascimento Saldiva PH, Mauad T, Marcia Negri E (2020) Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. https://doi.org/10.1111/jth.14844
Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S et al (2020) Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. https://doi.org/10.7326/M20-2003
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2015432
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395:1417–1418
McGonagle D, O’Donnell JS, Sharif K, Emery P, Bridgewood C (2020) Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. https://doi.org/10.1016/S2665-9913(20)30121-1
Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, Peccatori J, D'Angelo A, De Cobelli F, Rovere-Querini P, Tresoldi M, Dagna L, Zangrillo A (2020) Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc
Desborough MJR, Doyle AJ, Griffiths A, Retter A, Breen KA, Hunt BJ (2020) Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res. https://doi.org/10.1016/j.thromres.2020.05.049
Morel O, Marchandot B, Jesel L, Sattler L, Trimaille L, Curtiaud A, Ohana M, Fafi-Kremer S, Schini-Kerth V, Grunebaum L, Freyssinet JM. Microparticles in Covid-19 as a link between lung injury and thrombosis (in review)
Funding
No funding source in the writing of the manuscript and/or the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
Drafting of the manuscript, review, and editing, BM; drafting of the manuscript and critical revision for important intellectual content, AT; drafting of the manuscript, review, and critical revision for important intellectual content, AC; drafting of the manuscript, and critical revision for important intellectual content, KM; drafting of the manuscript, and critical revision for important intellectual content, LJ; drafting of the manuscript, and critical revision for important intellectual content, review, and supervision, OM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. All authors have read and approved submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marchandot, B., Trimaille, A., Curtiaud, A. et al. Thromboprophylaxis: balancing evidence and experience during the COVID-19 pandemic. J Thromb Thrombolysis 50, 799–808 (2020). https://doi.org/10.1007/s11239-020-02231-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02231-3