Abstract
Purpose
This clinimetric analysis was conducted to evaluate the reliability, validity, and responsiveness to changeover time of the QLQ-CIPN20 when used to quantify patient-reported chemotherapy-induced peripheral neuropathy (CIPN).
Methods
Participants recruited to four cooperative group trials were pooled to create two groups (n = 376, 575): those who did versus did not receive neurotoxic chemotherapy. QLQ-CIPN20 internal consistency reliability was assessed using Cronbach’s alpha coefficients. Instrument validity was assessed using factor analysis, by evaluating score correlations with other CIPN and pain measures, and by comparing scores between contrasting groups. Cohen’s d was used to assess responsiveness to change.
Results
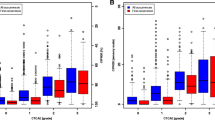
Alpha coefficients for the sensory, motor, and autonomic scales were 0.88, 0.88, and 0.78, respectively. However, autonomic scale and hearing loss items exhibited low item–item correlations (r ≤ 0.30) and thus were deleted. Moderate correlations were found between QLQ-CIPN20 and Brief Pain Inventory pain severity items (r 0.30–0.57, p ≤ .0001). Correlation between the QLQ-CIPN20 sensory and toxicity grading scale scores was low (r = .20; p ≤ .01). Mean scores were higher (worse) (p ≤ 0.0001) in individuals who did versus did not receive neurotoxic chemotherapy. The sensory and motor scales exhibited moderate-high responsiveness to change (Cohen’s d = 0.82 and 0.48, respectively). Factor analysis indicated that the 16-item version formed distinct factors for lower and upper extremity CIPN, delineating typical distal to proximal CIPN progression.
Conclusions
Results provide support for QLQ-CIPN20 sensory and motor scale reliability and validity. The more parsimonious and clinically relevant 16-item version merits further consideration.

Similar content being viewed by others
References
Hausheer, F. H., Schilsky, R. L., Bain, S., Berghorn, E. J., & Lieberman, F. (2006). Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Seminars in Oncology, 33(1), 15–49.
Argyriou, A. A., Bruna, J., Marmiroli, P., & Cavaletti, G. (2012). Chemotherapy-induced peripheral neurotoxicity (CIPN): An update. Critical Reviews in Oncology-Hematology, 82(1), 51–77.
Stubblefield, M. D., Burstein, H. J., Burton, A. W., Custodio, C. M., Deng, G. E., Ho, M., et al. (2009). NCCN task force report: Management of neuropathy in cancer. Journal of the National Comprehensive Cancer Network, 7(Suppl 5), S1–S26.
Argyriou, A. A., Zolota, V., Kyriakopoulou, O., & Kalofonos, H. P. (2010). Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents. Journal of B.U.On, 15(3), 435–446.
Paice, J. A. (2009). Clinical challenges: Chemotherapy-induced peripheral neuropathy. Seminars in Oncology Nursing, 25(2 Suppl 1), S8–S19.
Loprinzi, C. L., Reeves, B. N., Dakhil, S. R., Sloan, J. A., Wolf, S. L., Burger, K. N., et al. (2011). Natural history of paclitaxel-associated acute pain syndrome: Prospective cohort study NCCTG N08C1. Journal of Clinical Oncology, 29(11), 1472–1478.
Visovsky, C., & Daly, B. J. (2004). Clinical evaluation and patterns of chemotherapy-induced peripheral neuropathy. Journal of the American Academy of Nurse Practitioners, 16(8), 353–359.
Wampler, M. A., Miaskowski, C., Hamel, K., Byl, N., Rugo, H., & Topp, L. S. (2006). The modified total neuropathy score: A clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. Supportive Oncology, 4(8), W9–W16.
Bakitas, M. A. (2007). Background noise: The experience of chemotherapy-induced peripheral neuropathy. Nursing Research, 56(5), 323–331.
Smith, E. M. L., Bakitas, M. A., Homel, P., Piehl, M., Kingman, L., Fadul, C. E., et al. (2011). Preliminary assessment of a neuropathic pain treatment and referral algorithm for patients with cancer. Journal of Pain and Symptom Management, 42(6), 822–838.
Griffith, K., Merkies, I. S. J., Hill, E., & Cornblath, D. (2010). Measures of chemotherapy-induced peripheral neuropathy: A systematic review of psychometric properties. Journal of the Peripheral Nervous System, 15(4), 314–325.
Cavaletti, G., Frigeni, B., Lanzani, F., Mattavelli, L., Susani, E., Alberti, P., et al. (2010). Chemotherapy-Induced Peripheral Neurotoxicity assessment: A critical revision of the currently available tools. European Journal of Cancer, 46(3), 479–494.
Postma, T. J., Aaronson, N. K., Heimans, J. J., Muller, M. J., Hildebrand, J. G., Delattre, J. Y., et al. (2005). The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. European Journal of Cancer, 41(8), 1135–1139.
Cleeland, C. S., & Ryan, K. M. (1994). Pain assessment: Global use of the brief pain inventory. Annals of the Academy of Medicine, Singapore, 23(2), 129.
Common Terminology Criteria for Adverse Events Version 4.02 (CTCAE). (2009). U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 7 January, 2010.
Barton, D. L., Wos, E. J., Qin, R., Mattar, B. I., Green, N. B., Lanier, K. S., et al. (2011). A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Supportive care in Cancer: Official Journal of the Multinational Association of Supportive Care in Cancer, 19(6), 833–841.
Reeves, B. N., Dakhil, S. R., Sloan, J. A., Wolf, S. L., Burger, K. N., Kamal, A., et al. (2012). Further data supporting that the paclitaxel-associated acute pain syndrome is associated with the development of peripheral neuropathy: NCCTG Trial N08C1. Cancer, 118(20), 5171–5178.
Ferketich, S. (1991). Focus on psychometrics: Aspects of item analysis. Research in Nursing & Health, 14(2), 165–168.
Boehmer, S., & Luszczynska, A. (2006). Two kinds of items in quality of life instruments: ‘Indicator and causal variables’ in the EORTC qlq-c30. Quality of Life Research, 15(1), 131–141.
Fayers, P. M. (2004). Quality-of-life measurement in clinical trials—the impact of causal variables. Journal of Biopharmaceutical Statistics, 14(1), 155–176.
Pett, M. A., Lackey, N. R., & Sullivan, J. L. (2003). Making sense of factor analysis: The use of factor analysis for instrument development in health care research. Thousand Oaks: Sage Publications.
Barrett, P. P. (2007). Structural equation modeling: Adjudging model fit. Personality and Individual Differences, 42(5), 815–824.
Flora, D. D. B. (2004). An empirical evaluation of alternative methods of estimation for confirmatory factor analysis with ordinal data. Psychological Methods, 9(4), 466–491.
Hayduk, L. L. (2007). Testing! testing! one, two, three—Testing the theory in structural equation models! Personality and Individual Differences, 42(5), 841–850.
Beauducel, A. A. (2005). Simulation study on fit indexes in CFA based on data with slightly distorted simple structure. Structural Equation Modeling, 12(1), 41–75.
Fan, X. X. (2005). Sensitivity of fit indexes to misspecified structural or measurement model components: Rationale of two-index strategy revisited. Structural Equation Modeling, 12(3), 343–367.
Basch, E., Jia, X., Heller, G., Barz, A., Sit, L., Fruscione, M., et al. (2009). Adverse symptom event reporting by patients vs clinicians: Relationships With clinical outcomes. Journal of the National Cancer Institute, 101(23), 1624–1632.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Hillsdale, New Jersey: Lawrence Earlbaum and Associate.
Clayton, M. F., & Pett, M. A. (2011). Modeling relationships in clinical research using path analysis Part II: Evaluating the model. Journal for Specialists in Pediatric Nursing: JSPN, 16(1), 75–79.
Hu, L. L. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6(1), 1–55.
Zheng, Z. E. (2010). Research note–toward a causal interpretation from observational data: A new bayesian networks method for structural models with latent variables. Information Systems Research, 21(2), 365–391.
Spirtes, P., Glymour, C., Scheines, R., & Tillman, R. (2010). Automated search for causal relations: Theory and practice. In R. Dechter, H. Geffner, & J. Y. Halpern (Eds.), Heuristics, probability, and causality: A tribute to judea pearl (pp. 467–506). London, UK: College Publications.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-124477. CA-63848, CA-52352, CA-35090, CA-35101, CA-35269, CA-37417, CA-35448, CA-63844, CA-35267, CA-35272, CA-35113, CA-35103, CA-35415, and CA-35431. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health. A special thanks is extended to Suneetha Puttabasavaiah, BS for her assistance in data management.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lavoie Smith, E.M., Barton, D.L., Qin, R. et al. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual Life Res 22, 2787–2799 (2013). https://doi.org/10.1007/s11136-013-0379-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-013-0379-8