Abstract
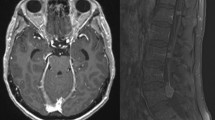
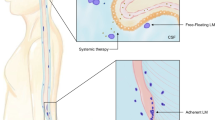
Leptomeningeal metastasis is a devastating complication of the central nervous system in patients with late-stage solid or hematological cancers. Leptomeningeal metastasis results from the multifocal seeding of the leptomeninges by malignant cancer cells. Although central nervous system metastasis usually presents in patients with widely disseminated and progressive late-stage cancer, malignant cells may spread to the cerebrospinal fluid during earlier disease stages in particularly aggressive cancers. Treatment of leptomeningeal metastasis is largely palliative but will often provide stabilization and protection from further neurological deterioration and improve quality of life. There is a need to raise awareness of the impact of leptomeningeal metastases on cancer patients and its known and putative biological basis. Novel diagnostic approaches include identification of biomarkers that may stratify the risk for developing leptomeningeal metastasis. Current therapies can be used more effectively while waiting for advanced treatments to be developed.


Similar content being viewed by others
References
Kesari S, Batchelor TT (2003) Leptomeningeal metastases. Neurol Clin N Am 21:25–66
Gleissner B, Chamberlain MC (2006) Neoplastic meningitis. Lancet Neurol 5:443–452
Groves MD, Hess KR, Puduvalli VK et al (2009) Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J Neurooncol 4:229–234
Mitchell MS (1989) Relapse in the central nervous system in melanoma patients successfully treated with biomodulators. J Clin Oncol 7:1701–1709
O’Meara WP, Borkar SA, Stambuk HE et al (2007) Leptomeningeal metastasis. Curr Probl Cancer 31:372–424
Theodore WH, Gendelman S (1981) Meningeal carcinomatosis. Arch Neurol 38:696–699
Yap HY, Yap BS, Rasmussen S et al (1982) Treatment for meningeal carcinomatosis in breast cancer. Cancer 50:219–222
Jorda M, Ganjei-Azar P, Nadji M (1998) Cytologic characteristics of meningeal carcinomatosis. Increased diagnostic accuracy using carcinoembryonic antigen and epithelial membrane antigen immunocytochemistry. Arch Neurol 55:181–184
Chamberlain MC (2006) CSF Disseminated primary brain tumors. In: Newton HB (ed) Handbook of brain tumor chemotherapy. Elsevier Science, San Diego, pp 316–326
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5–14
Glass JP, Melamed M, Chernik NL et al (1979) Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology 29:1369–1375
Gleissner B, Chamberlain MC (2007) Treatment of CNS dissemination in systemic lymphoma. J Neurooncol 84:107–117
Hatton C (2005) Lymphomatous meningitis. Haematologica Rep 1:108–109
Chamberlain MC (2008) Neoplastic meningitis. Curr Neurol Neurosci Rep 8:249–258
Yoshida S, Morii K, Watanabe M et al (2000) Characteristic features of malignant lymphoma with central nervous system involvement. Surg Neurol 53:163–167
Glantz MJ, Cole BF, Glantz LK et al (1998) Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 82:733–739
Ward MS (1999) The use of flow cytometry in the diagnosis and monitoring of malignant hematological disorders. Pathology 31:382–392
Johanson CE, Duncan JA, Stopa EG et al (2005) Enhanced prospects for drug delivery and brain targeting by the choroid plexus-CSF route. Pharm Res 22:1011–1037
Kobayashi Z, Tsuchiya K, Machida A et al (2009) Metastatic CNS lymphoma presenting with periventricular dissemination–MRI and neuropathological finding in an autopsy case. J Neurological Sci 277:109–113
Levine S (1987) Choroid plexus: target for systemic disease and pathway to the brain. Lab Invest 56:231–233
Oldstone MB, Southern PJ (1993) Trafficking of activated cytotoxic T lymphocytes into the central nervous system: use of a transgenic model. J Neuroimmunol 46:25–31
Groves MD (2003) The pathogenesis of neoplastic meningitis. Curr Oncol Rep 5:15–23
Alix-Panabieres C, Riethdorf S, Pantel K (2008) Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res 14:5013–5021
Kakarala M, Wicha MS (2008) Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol 26:2813–2820
Li X, Lewis MT, Huang J et al (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100:672–679
Fidler IJ, Langley RR, Kerbel RS et al (2005) Angiogenesis. In: De Vita VT, Hellman S, Rosenberg SA (eds) Cancer: principles and practice in oncology. Lippincott Williams & Wilkins, New York, pp 129–135
Kraft A, Weindel K, Ochs A et al (1999) Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer 85:178–187
Stockhammer G, Poewe W, Burgstaller S et al (2000) Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology 54:1670–1676
Goswami S, Phillippar U, Sun D et al (2009) Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin Exp Metastasis 26:153–159
Kadoch C, Treseler P, Rubenstein JL (2006) Molecular pathogenesis of primary central nervous system lymphoma. Neurosurg Focus 21:E1
Lee B-C, Lee T-H, Avraham S et al (2004) Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res 2:327–338
Singh S, Nannuru KC, Sandanandam A et al (2009) CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. Br J Cancer 100:1638–1646
Philippar U, Roussos ET, Oser M et al (2008) A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 15:813–828
Wyckoff J, Wang W, Lin EY et al (2004) A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 64:7022–7029
Van Oostenbrugge RJ, Hopman AHN, Ramaekers FCS et al (1998) In situ hybridization: a possible diagnostic aid in leptomeningeal metastasis. J Neurooncol 38:127–133
Van Oostenbrugge RJ, Hopman AHN, Arends JW et al (2000) Treatment of leptomeningeal metastasis evaluated by interphase cytogenetics. J Clin Oncol 18:2053–2058
Baehring JM, Hochberg FH, Betensky RA et al (2006) Immunoglobulin gene rearrangement analysis in cerebrospinal fluid of patients with lymphoproliferative processes. J Neurol Sci 247:208–216
Gleissner B, Siehl J, Korfel A et al (2002) CSF evaluation in primary CNS lymphoma patients by PCR of the CDR III IgH genes. Neurology 58:390–396
Berg SL, Chamberlain MC (2003) Systemic chemotherapy, intrathecal chemotherapy, and symptom management in the treatment of leptomeningeal metastasis. Curr Oncol Rep 5:29–40
Berg SL, Chamberlain MC (2005) Current treatment of leptomeningeal metastases: systemic chemotherapy, intrathecal chemotherapy and symptom management. Cancer Treat Res 125:121–146
Chamberlain MC, Nolan C, Abrey LE (2005) Leukemic and lymphomatous meningitis: incidence, prognosis and treatment. J Neurooncol 75:71–83
Chamberlain MC, Kormanik PA, Barba D (1997) Complications associated with intraventricular chemotherapy in patients with leptomeningeal metastases. J Neurosurg 87:694–699
Siegal T, Lossos A, Pfeffer MR (1994) Leptomeningeal metastases: analysis of 31 patients with sustained off-therapy response following combined-modality therapy. Neurology 44:1463–1469
Glantz MJ, Hall WA, Cole BF et al (1995) Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer 75:2919–2931
Glantz MJ, Hall WA, Cole BF et al (1995) Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer 75(12):2919–2931
Chamberlain MC, Glantz M (2006) Ventriculoperitoneal shunt in patients with leptomeningeal metastases. Neurology 66:783
Omuro AM, Lallana EC, Bilsky MH et al (2005) Ventriculoperitoneal shunt in patients with leptomeningeal metastases. Neurology 10:1625–1627
Mocco J, Tomey MI, Komotar RJ et al (2006) Ventriculoperitoneal shunting of idiopathic normal pressure hydrocephalus increases midbrain size: a potential mechanism for gait improvement. Neurosurgery 59:847–851
Schiff D, Kline C, Meltzer H et al (2009) Palliative venticuloperitoneal shunt in a pediatric patient with recurrent metastatic medulloblastoma. J Palliat Med 12:391–393
Lokich J, Levine H, Nasser I (1998) Malignancy-related hydrocephalus: clinical features and results of ventricular peritoneal shunt procedure in three patients. Am J Clin Oncol 21:366–368
Eralp Y, Saip P, Aydin Z et al (2008) Leptomeningeal dissemination of ovarian carcinoma through a ventriculoperitoneal shunt. Gynecol Oncol 108:248–250
Matsumae M, Sato O, Itoh K et al (1989) Quantification of cerebrospinal fluid shunt flow rates. Assessment of the programmable pressure valve. Childs Nerv Syst 5:356–360
Chiafery M (2006) Care and management of the child with shunted hydrocephalus. Pediatr Nurs 32:222–225
Glantz MJ, LaFollette S, Jaeckle KA et al (1999) Randomized trial of a slow-release versus standard formulation of cytarabine for the intrathecal treatment of lymphomatous meningitis. J Clin Oncol 17:3110–3116
Garcia-Marco JA, Panizo C, Garcia ES et al (2009) Efficacy and safely of liposomal cytarabine in lymphoma patients with central nervous system involvement from lymphoma. Cancer 115:1892–1898
Adams GP, Weiner LM (2005) Monoclonal antibody therapy of cancer. Nat Biotechnol 23:1147–1157
Cameron DA, Stein S (2008) Drug insight: intracellular inhibitors of HER2—clinical development of lapatinib in breast cancer. Nat Clin Pract Oncol 5:512–520
Lin NU, Diéras V, Paul D et al (2009) Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 15:1452–1459
Onishi H, Morisaki T, Nakafusa Y et al. (2011) Objective response with lapatinib in patients with meningitis carcinomatosa derived from HER2/HER1-negative breast cancer. Int J Clin Oncol. doi:10.1007/s10147-011-0195-5
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Inoue A, Kobayashi K, Usui K et al (2009) First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol 27:1394–1400
Omuro AM, Kris MG, Miller VA et al (2005) High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 103:2344–2348
Jackman DM, Kesari S, Cioffredi L et al. (2009) Phase I study of high dose gefitinib in patients with leptomeningeal metastases from EGFR-mutant non-small cell lung cancer. Presented at the 13th World Congress on Lung Cancer San Francisco, 31st July–4th August 2009; Poster P1.189
Yi HG, Kim HJ, Kim YJ et al (2009) Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are effective for leptomeningeal metastasis from non-small cell lung cancer patients with sensitive EGFR mutation or other predictive factors of good response for EGFR TKI. Lung Cancer 65:80–84
Jackman DM, Holmes A, Lindeman N et al (2006) Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol 24:4517–4520
Clarke JL, Pao W, Wu N et al (2010) High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 99(2):283–286
Tetsumoto S, Osa A, Kijima T et al (2011) Two cases of leptomeningeal metastases from lung adenocarcinoma which progressed during gefitinib therapy but responded to erlotinib. Int J Clin Oncol. doi:10.1007/s10147-011-0256-9
Shigekawa T, Takeuchi H, Misumi M et al (2009) Successful treatment of leptomeningeal metastases from breast cancer using the combination of trastuzumab and capecitabine: a case report. Breast Cancer 16:88–92
Ekenel M, Hormigo AM, Peak S et al (2007) Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol 85:223–227
Tham YL, Hinckley L, Teh BS et al (2006) Long-term clinical response in leptomeningeal metastases from breast cancer treated with capecitabine monotherapy: a case report. Clin Breast Cancer 7:164–166
Paydas S, Bicakci K, Yavuz S (2009) Dramatic response with capecitabine after cranial radiation to the brain parenchymal and leptomeningeal metastases from lung cancer. Eur J Intern Med 20:96–99
Rivera E, Meyers C, Groves M et al (2006) Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer 107:1348–1354
Fidler IJ, Yano S, Zhang RD et al (2002) The seed and soil hypothesis: vascularization and brain metastases. Lancet Oncol 3:53–57
Bhaskara A, Eng C (2008) Bevacizumab in the treatment of a patient with metastatic colorectal carcinoma with brain metastases. Clin Colorectal Cancer 7:65–68
Kim LS, Huang S, Lu W et al (2004) Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis 21:107–118
Posner JB (1996) Brain metastases: 1995 a brief review. J Neurooncol 27:287–293
Ku GY, Krol G, Ilson DH (2007) Successful treatment of leptomeningeal disease in colorectal cancer with a regimen of bevacizumab, temozolomide, and irinotecan. J Clin Oncol 25:e14–e16
Cvetkovic RS, Perry CM (2006) Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Drugs 66:791–820
Marcus R, Hagenbeek A (2007) The therapeutic use of rituximab in non-Hodgkin’s lymphoma. Eur J Haematol Suppl 67:5–14
Rubenstein JL, Fridlyand J, Abrey L et al (2007) Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 25:1350–1355
Chamberlain MC, Johnston SK, Van Horn A et al (2009) Recurrent lymphomatous meningitis treated with intra-CSF rituximab and liposomal ara-C. J Neurooncol 91:271–277
Bernstein SH, Unger JM, Leblanc M et al (2009) Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516—the southwest oncology group. J Clin Oncol 27:114–119
Canales M, Peñarrubia MJ, Salar A et al. (2008) Efficacy and safety of intrathecal liposomal cytarabine injection given prophylactically in patients with high-risk diffuse large B-cell lymphoma: a report of 24 patients in Spain. Abstract Presented at: the 13th Congress of the European Hematology Association, Copenhagen, Denmark, 12–15 June
Lim HY, Thiel E, Glantz MJ (2008) To protect and defend: central nervous system prophylaxis in patients with non-Hodkin’s lymphoma. Curr Opin Oncol 20:495–501
Acknowledgment
This work was supported in part by grants from NIH (NIH 3P30CA023100-25S8) to S. Kesari.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grewal, J., Saria, M.G. & Kesari, S. Novel approaches to treating leptomeningeal metastases. J Neurooncol 106, 225–234 (2012). https://doi.org/10.1007/s11060-011-0686-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0686-2