Abstract
Objectives
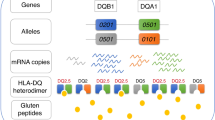
Gastrointestinal manifestations are frequent in patients with common variable immunodeficiency (CVID), and some of the patients present with celiac-like features. Diagnosing celiac disease (CD) in CVID however is challenging, as autoantibody detection and histopathology of the small intestine cannot reliably discriminate between classic CD and a celiac-like disease in these individuals. For the development of classic gluten-sensitive CD a certain HLA haplotype involving the loci DQA1* and DQB1* and encoding two different HLA DQ heterodimers is the prerequisite. We aimed to determine the frequency of these haplotypes in CVID patients with suspected CD. Furthermore, we report on autoimmune manifestations and the lymphocyte phenotype in these patients.
Methods
By retrospective analysis data on gastrointestinal symptoms, diet, concurrent autoimmune diseases, and routine laboratory values were collected. CVID patients were classified according to their B-cell phenotype. Expression of HLA-DQA1* and HLA-DQB1* alleles were determined by genetic analysis.
Results
Twenty out of 250 CVID patients presented with a clinical phenotype resembling celiac disease. Four (20 %) out of these CVID patients carried the CD-associated HLA DQ2.5 or DQ8 heterodimer, while HLA DQ2.5 was present in 100 % of a CD control cohort. Gluten-free diet (GFD) resulted in a clinical and histological response in two out of four patients with HLA high-risk alleles for CD. The response could not be assessed in the remaining two patients, as these patients did not adhere sufficiently long to GFD. The percentage of autoimmune manifestations other than CD was high (50 %) in CVID patients presenting with a CD-like enteropathy, and most of these patients had an expansion of B-cells with low expression of CD21 (CD21low B-cells).
Conclusions
In CVID patients with suspected celiac disease typing of the HLA loci DQA1 and DQB1 can help to identify those that have a genetic susceptibility for CD. In CVID patients with a celiac-like phenotype but negative for CD-associated HLA-DQ markers, an autoimmune enteropathy (AIE) as part of an extended autoimmune dysregulation needs to be considered. This has important implications for further diagnostics and therapy of these patients.
Similar content being viewed by others
References
Agarwal S, Cunningham-Rundles C. Autoimmunity in common variable immunodeficiency. Curr Allergy Asthma Rep. 2009;9:347–52.
Teahon K, Webster AD, Price AB, Weston J, Bjarnason I. Studies on the enteropathy associated with primary hypogammaglobulinaemia. Gut. 1994;35:1244–9.
Khodadad A, Aghamohammadi A, Parvaneh N, Rezaei N, Mahjoob F, Bashashati M, et al. Gastrointestinal manifestations in patients with common variable immunodeficiency. Dig Dis Sci. 2007;52:2977–83.
Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–16.
Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol. 1996;20:1240–52.
Kalha I, Sellin JH. Common variable immunodeficiency and the gastrointestinal tract. Curr Gastroenterol Rep. 2004;6:377–83.
Luzi G, Zullo A, Iebba F, Rinaldi V, Sanchez Mete L, Muscaritoli M, et al. Duodenal pathology and clinical-immunological implications in common variable immunodeficiency patients. Am J Gastroenterol. 2003;98:118–21.
Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60.
Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48.
Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469–77.
Fallang L-E, Bergseng E, Hotta K, Berg-Larsen A, Kim C-Y, Sollid LM. Differences in the risk of celiac disease associated with HLA-DQ2.5 or HLA-DQ2.2 are related to sustained gluten antigen presentation. Nat Immunol. 2009;10:1096–101.
Bodd M, Kim C-Y, Lundin KEA, Sollid LM. T-cell response to gluten in patients with HLA-DQ2.2 reveals requirement of peptide-MHC stability in celiac disease. Gastroenterology. 2012;142:552–61.
Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93:190–7.
American Gastroenterological Association medical position statement: guidelines for the evaluation and management of chronic diarrhea. Gastroenterology. 1999; 116:1461–1463.
Biagi F, Bianchi PI, Zilli A, Marchese A, Luinetti O, Lougaris V, et al. The significance of duodenal mucosal atrophy in patients with common variable immunodeficiency: a clinical and histopathologic study. Am J Clin Pathol. 2012;138:185–9.
Warnatz K. Severe deficiency of switched memory B cells (CD27 + IgM-IgD-) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51.
Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85.
Marsh SGE. Nomenclature for factors of the HLA system, update September 2012. Tissue Antigens. 2012;80:564–9.
Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94.
Ahn R, Ding YC, Murray J, Fasano A, Green PHR, Neuhausen SL, et al. Association analysis of the extended MHC region in celiac disease implicates multiple independent susceptibility loci. PLoS One. 2012;7:e36926.
Malamut G, Verkarre V, Suarez F, Viallard J-F, Lascaux A-S, Cosnes J, et al. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol. 2010;105:2262–75.
Oksenhendler E, Gérard L, Fieschi C, Malphettes M, Mouillot G, Jaussaud R, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008;46:1547–54.
Agarwal S, Smereka P, Harpaz N, Cunningham-Rundles C, Mayer L. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm Bowel Dis. 2011;17:251–9.
Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–94.
Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36.
Mesin L, Sollid LM, Di Niro R. The intestinal B-cell response in celiac disease. Front Immunol. 2012;3:313.
Megiorni F, Mora B, Bonamico M, Barbato M, Nenna R, Maiella G, et al. HLA-DQ and risk gradient for celiac disease. Hum Immunol. 2009;70:55–9.
Megiorni F, Pizzuti A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing. J Biomed Sci. 2012;19:88.
Harmon GS, Lebeck LK, Weidner N. Gluten-dependent enteropathy and atypical human leukocyte antigen alleles. Hum Pathol. 2011;42:1112–6.
Trynka G, Wijmenga C, van Heel DA. A genetic perspective on coeliac disease. Trends Mol Med. 2010;16:537–50.
Dubois PCA, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302.
Garner CP, Murray JA, Ding YC, Tien Z, van Heel DA, Neuhausen SL. Replication of celiac disease UK genome-wide association study results in a US population. Hum Mol Genet. 2009;18:4219–25.
Hunt KA, Zhernakova A, Turner G, Heap GAR, Franke L, Bruinenberg M, et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008;40:395–402.
van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova A, Inouye M, et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet. 2007;39:827–9.
Kaukinen K, Collin P, Mykkänen AH, Partanen J, Mäki M, Salmi J. Celiac disease and autoimmune endocrinologic disorders. Dig Dis Sci. 1999;44:1428–33.
Ventura A, Magazzù G, Greco L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology. 1999;117:297–303.
Wehr C, Eibel H, Masilamani M, Illges H, Schlesier M, Peter H-H, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113:161–71.
Acknowledgments
We thank the patients participating in this study. This study was supported by the Federal Ministry of Education and Research (BMBF 01EO 0803). The authors are responsible for the contents of this publication.
Conflict of Interest
The authors have to conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Florian Emmerich and Michaela Neagu contributed equally
Rights and permissions
About this article
Cite this article
Venhoff, N., Emmerich, F., Neagu, M. et al. The Role of HLA DQ2 and DQ8 in Dissecting Celiac-Like Disease in Common Variable Immunodeficiency. J Clin Immunol 33, 909–916 (2013). https://doi.org/10.1007/s10875-013-9892-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-013-9892-3