Abstract
Introduction
Sarcoidosis is an inflammatory disease of unknown etiology. However, an infectious cause has been proposed suggesting a role for pattern-recognition receptors, such as Toll-like receptors (TLRs) and nucleotide-binding domain, leucin-rich repeat containing family proteins (NLRs), in the pathogenesis.
Objective
Our aim was to investigate whether differences in TLR2 and TLR4 expression, and the response to TLR2, TLR4, and NOD2 stimulation, are associated with sarcoidosis.
Materials and Methods
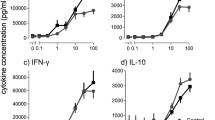
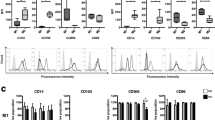
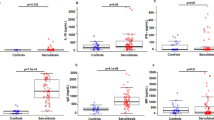
Blood mononuclear cells from sarcoidosis patients (n = 24) and healthy subjects (n = 19) were incubated with the TLR2 ligands PGN and Pam3CSK4, the TLR4 ligand LPS, the NOD2 ligand MDP, or medium alone. After 16 h, monocyte TLR2 and TLR4 expression and cytokine secretion, including TNFα, IL-1β, IL-6, IL-8, IL-10, and IL-12p70, were measured using flow cytometry and cytometric bead array.
Results
TLR2 and TLR4 expression at baseline was significantly higher in patients. Combined TLR2 and NOD2 stimulation induced a four-fold higher secretion of TNFα and a 13-fold higher secretion of IL-1β in patients. Additionally, there was a synergistic effect of TLR2 with NOD2 stimulation on induction of IL-1β in patients, whereas IL-10 was synergistically induced in healthy subjects.
Conclusion
Increased TLR expression and enhanced secretion of pro-inflammatory cytokines after combined TLR2 and NOD2 stimulation may be related to the pathogenesis of sarcoidosis.







Similar content being viewed by others
References
Parkes SA, Baker SB, Bourdillon RE, Murray CR, Rakshit M. Epidemiology of sarcoidosis in the Isle of Man—1: a case controlled study. Thorax 1987;42(6):420–6.
Hosoda Y, Yamaguchi M, Hiraga Y. Global epidemiology of sarcoidosis. What story do prevalence and incidence tell us? Clin Chest Med 1997;18(4):681–94. doi:10.1016/S0272-5231(05)70412-3.
Saboor SA, Johnson NM, McFadden J. Detection of mycobacterial DNA in sarcoidosis and tuberculosis with polymerase chain reaction. Lancet 1992;339(8800):1012–5. doi:10.1016/0140-6736(92)90535-B.
Abe C, Iwai K, Mikami R, Hosoda Y. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl Bakteriol Mikrobiol Hyg [A] 1984;256(4):541–7.
Mitchell JA, Paul-Clark MJ, Clarke GW, McMaster SK, Cartwright N. Critical role of toll-like receptors and nucleotide oligomerisation domain in the regulation of health and disease. J Endocrinol 2007;193(3):323–30. doi:10.1677/JOE-07-0067.
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411(6837):603–6. doi:10.1038/35079114.
Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, et al. CARD15 mutations in Blau syndrome. Nat Genet 2001;29(1):19–20. doi:10.1038/ng720.
Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S, et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 2005;105(3):1195–7. doi:10.1182/blood-2004-07-2972.
Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 2003;278(8):5509–12. doi:10.1074/jbc.C200673200.
Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol 2004;16(1):35–41. doi:10.1016/j.smim.2003.10.005.
Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 2005;1(3):279–85. doi:10.1371/journal.ppat.0010034.
Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol 2005;124(5):931–8. doi:10.1111/j.0022-202X.2005.23705.x.
Tanabe T, Ishige I, Suzuki Y, Aita Y, Furukawa A, Ishige Y, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim Biophys Acta 2006;1762(9):794–801.
Most J, Neumayer HP, Dierich MP. Cytokine-induced generation of multinucleated giant cells in vitro requires interferon-gamma and expression of LFA-1. Eur J Immunol 1990;20(8):1661–7. doi:10.1002/eji.1830200807.
Mizuno K, Okamoto H, Horio T. Heightened ability of monocytes from sarcoidosis patients to form multi-nucleated giant cells in vitro by supernatants of concanavalin A-stimulated mononuclear cells. Clin Exp Immunol 2001;126(1):151–6. doi:10.1046/j.1365-2249.2001.01655.x.
Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 1999;160(2):736–55.
Lofgren S, Lundback H. The bilateral hilar lymphoma syndrome; a study of the relation to tuberculosis and sarcoidosis in 212 cases. Acta Med Scand 1952;142(4):265–73.
Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, et al. Mycobacterial catalase–peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005;201(5):755–67. doi:10.1084/jem.20040429.
Jones BW, Means TK, Heldwein KA, Keen MA, Hill PJ, Belisle JT, et al. Different Toll-like receptor agonists induce distinct macrophage responses. J Leukoc Biol 2001;69(6):1036–44.
Philpott DJ, Girardin SE. The role of Toll-like receptors and Nod proteins in bacterial infection. Mol Immunol 2004;41(11):1099–108. doi:10.1016/j.molimm.2004.06.012.
Veltkamp M, Grutters JC, van Moorsel CH, Ruven HJ, van den Bosch JM. Toll-like receptor (TLR) 4 polymorphism Asp299Gly is not associated with disease course in Dutch sarcoidosis patients. Clin Exp Immunol 2006;145(2):215–8. doi:10.1111/j.1365-2249.2006.03127.x.
Veltkamp M, Wijnen PA, van Moorsel CH, Rijkers GT, Ruven HJ, Heron M, et al. Linkage between Toll-like receptor (TLR) 2 promotor and intron polymorphisms: functional effects and relevance to sarcoidosis. Clin Exp Immunol 2007;149(3):453–62.
Schurmann M, Kwiatkowski R, Albrecht M, Fischer A, Hampe J, Muller-Quernheim J, et al. Study of Toll-like receptor gene loci in sarcoidosis. Clin Exp Immunol 2008;152(3):423–31.
Lauener RP, Birchler T, Adamski J, Braun-Fahrlander C, Bufe A, Herz U, et al. Expression of CD14 and Toll-like receptor 2 in farmers’ and non-farmers’ children. Lancet 2002;360(9331):465–6. doi:10.1016/S0140-6736(02)09641-1.
Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res 2005;6:68. doi:10.1186/1465-9921-6-68.
Valeyre D, Soler P, Clerici C, Pre J, Battesti JP, Georges R, et al. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax 1988;43(7):516–24.
Homma T, Kato A, Hashimoto N, Batchelor J, Yoshikawa M, Imai S, et al. Corticosteroid and cytokines synergistically enhance toll-like receptor 2 expression in respiratory epithelial cells. Am J Respir Cell Mol Biol 2004;31(4):463–9. doi:10.1165/rcmb.2004-0161OC.
Bosisio D, Polentarutti N, Sironi M, Bernasconi S, Miyake K, Webb GR, et al. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood 2002;99(9):3427–31. doi:10.1182/blood.V99.9.3427.
Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum 2004;50(5):1457–67. doi:10.1002/art.20219.
Jendraschak E, Kaminski WE, Kiefl R, von Schacky C. IGF-1, PDGF and CD18 are adherence-responsive genes: regulation during monocyte differentiation. Biochim Biophys Acta 1998;1396(3):320–35.
Krause SW, Kreutz M, Andreesen R. Differential effects of cell adherence on LPS-stimulated cytokine production by human monocytes and macrophages. Immunobiology 1996;196(5):522–34.
Martin TM, Doyle TM, Smith JR, Dinulescu D, Rust K, Rosenbaum JT. Uveitis in patients with sarcoidosis is not associated with mutations in NOD2 (CARD15). Am J Ophthalmol 2003;136(5):933–5. doi:10.1016/S0002-9394(03)00892-4.
Gazouli M, Koundourakis A, Ikonomopoulos J, Gialafos EJ, Rapti A, Gorgoulis VG, et al. CARD15/NOD2, CD14, and toll-like receptor 4 gene polymorphisms in Greek patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006;23(1):23–9.
Iannuzzi MC. Advances in the genetics of sarcoidosis. Proc Am Thorac Soc 2007;4(5):457–60. doi:10.1513/pats.200606-136MS.
Strausz J, Mannel DN, Pfeifer S, Borkowski A, Ferlinz R, Muller-Quernheim J. Spontaneous monokine release by alveolar macrophages in chronic sarcoidosis. Int Arch Allergy Appl Immunol 1991;96(1):68–75.
Idali F, Wiken M, Wahlstrom J, Mellstedt H, Eklund A, Rabbani H, et al. Reduced Th1 response in the lungs of HLA-DRB1*0301 patients with pulmonary sarcoidosis. Eur Respir J 2006;27(3):451–9. doi:10.1183/09031936.06.00067105.
Berlin M, Fogdell-Hahn A, Olerup O, Eklund A, Grunewald J. HLA-DR predicts the prognosis in Scandinavian patients with pulmonary sarcoidosis. Am J Respir Crit Care Med 1997;156:1601–5.
Uehara A, Yang S, Fujimoto Y, Fukase K, Kusumoto S, Shibata K, et al. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell Microbiol 2005;7(1):53–61. doi:10.1111/j.1462-5822.2004.00433.x.
Netea MGFG, de Jong DJ, Jansen T, Jacobs L, Kramer M, Naber TH, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol 2005;174(10):6518–23.
Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 2004;5(8):800–8. doi:10.1038/ni1092.
Kramer M, Netea MG, de Jong DJ, Kullberg BJ, Adema GJ. Impaired dendritic cell function in Crohn’s disease patients with NOD2 3020insC mutation. J Leukoc Biol 2006;79(4):860–6. doi:10.1189/jlb.0805484.
Werts C, Girardin SE, Philpott DJ. TIR, CARD and PYRIN: three domains for an antimicrobial triad. Cell Death Differ 2006;13(5):798–815. doi:10.1038/sj.cdd.4401890.
Acknowledgements
The authors thank Margitha Dahl, Gunnel de Forest, Heléne Blomqvist, and Berit Olsson for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Swedish Heart–Lung Foundation, King Oscar II Jubilee Foundation, the Swedish Research Council, the U.S. National Institutes of Health (Grant No. 1 R21 HL077579-01), the Stockholm County Council and Karolinska Institutet.
Electronic supplementary material
Below is the link to the electronic supplementary material
Table EI
Characterization of Patient Subgroups (DOC 52.0 KB)
Figure E1
TLR2 (A) and TLR4 (B) expression on peripheral blood monocytes of sarcoidosis patients with Löfgren’s syndrome (white boxes) and non-Löfgren’s syndrome (grey boxes) after in vitro stimulation with ligands indicated on the x-axis. Boxes show median and interquartile range, whiskers show range. Numbers below the boxes state the number of patients and controls in each analysis. Numbers within the parenthesis state the number of Löfgren’s syndrome patients and non-Löfgren’s syndrome patients, respectively, in each analysis (DOC 32.0 KB).
Figure E2
Secretion of TNFα (A), IL-1β (B), IL-6 (C), IL-8 (D) and IL-10 (E) from human mononuclear cells of sarcoidosis patients with Löfgren’s syndrome (white boxes) and non-Löfgren’s syndrome (grey boxes) after stimulation with ligands indicated on the x-axis. Boxes show median and interquartile range, whiskers show range. Numbers below the boxes state the number of patients and controls in each analysis. Numbers within the parenthesis state the number of Löfgren’s syndrome patients and non-Löfgren’s syndrome patients, respectively, in each analysis (DOC 38.0 KB).
Figure E3
Representative histograms of flow cytometric analysis of TLR2 (a) and TLR4 (b), after stimulation with various ligands (DOC 375 KB).
Rights and permissions
About this article
Cite this article
Wikén, M., Grunewald, J., Eklund, A. et al. Higher Monocyte Expression of TLR2 and TLR4, and Enhanced Pro-inflammatory Synergy of TLR2 with NOD2 Stimulation in Sarcoidosis. J Clin Immunol 29, 78–89 (2009). https://doi.org/10.1007/s10875-008-9225-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-008-9225-0