Abstract
The relation between body mass index (BMI) and risk of cancer incidence is controversial. Cancer incidence during 1972–2008 in relation to BMI was investigated in a prospective cohort of 54,725 Finns aged 24–74 years and free of cancer at enrollment. Over a mean follow-up of 20.6 years, 8,429 (15.4 %) incident cancers were recorded, 4,208 (49.9 %) from men. Both parametric and nonparametric approaches were used to evaluate the shape of the relationship between BMI and incidence of cancer. BMI had a linear positive association with incidence of cancers of the colon, liver, kidney, bladder and all sites combined in men, and of cancers of the stomach, colon, gallbladder and ovary in women, an inverse association with incidence of cancers of the lung in men and the lung and breast in women, a J-shaped association with incidence of all cancers combined in women. High BMI in women was associated with an increased overall cancer risk in never smokers but a reduced risk in smokers. Elevated BMI was associated with an increased risk of incidence of cancers of certain sites.
Similar content being viewed by others
Introduction
The prevalence of obesity has dramatically increased for decades worldwide. Body mass index (BMI) is one of the most commonly used surrogate measurements of overweight (BMI 25.0–29.9 kg/m2) and obesity (BMI ≥ 30.0 kg/m2) [1]. The majority of studies supported the hypothesis that elevated BMI was associated with an increased risk of incidence of cancers of the colon [2–7], pancreas [3, 8–12] and kidney [6, 10, 13–15], but with a decreased risk of incidence of lung cancer [6, 10, 16–18]. No association was found between BMI and incidence of cancers of the prostate [19–25] and rectum [4, 5, 26–29]. But, the relationship between BMI and incidence of cancers of other sites is still inconsistent: a linear positive relationship [30, 31] or no [6] relationship with gallbladder cancer, an inverse relationship [6] or no [10, 32] relationship with stomach cancer, a non-significant positive association with the liver [3, 6, 33, 34], cervix [3, 10, 33] or all sites combined [3, 6, 10], and a linear positive relationship [35] or no relationship [3, 6, 10, 36] with the bladder. Recent evidence also suggests that obesity is associated with an increased risk of incidence of ovarian cancer [10, 37–40]. It is generally accepted that elevated BMI is associated with a decreased risk of incidence of breast cancer for premenopausal women, but the relationship is reversed in postmenopausal women [3, 10, 41–47].
We investigated the shape of the relationship between BMI and incidence of cancers in a large prospective Finnish cohort.
Methods
Study population
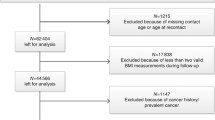
Population-based surveys on CVD and other non-communicable disease risk factors and their developing trends have been conducted every 5 years since 1972 in Finland (FINRISK study) [48]. Seven FINRISK cohorts of 1972, 1977, 1982, 1987, 1992, 1997 and 2002 are included in the current data analysis. All seven surveys included subjects who were 24–64 years of age, and the 1997 and 2002 survey also included subjects aged 65–74 years. The total number of persons in the seven surveys was 60,504, including 29,401 men and 31,103 women. The FINRISK studies were approved by the Ethics Committee of the National Public Health Institute, Helsinki, Finland.
Baseline measurements
The surveys included a self-administered questionnaire (mainly including questions on socioeconomic factors, medical history, health behaviour, and psychosocial factors), physical examinations and laboratory measures. Height and weight were measured on site by specially trained nurses with participants not wearing shoes and heavy clothing. BMI was calculated as body weight in kilograms divided by the square of height in meters. Smoking status was classified into three categories (never smokers, former smokers and current smokers). Leisure-time physical activity was graded in three categories (sedentary: reading, watching television, or other sedentary activity; mild: walking, bicycling or exercise otherwise at least 4 h per week; intensive: running, jogging, skiing, swimming, or heavy garden work, etc., at least 3 h per week, or participating in athletic competitions or regularly exercising several times a week). Education was classified into three categories (≤9, 10–12, >13 schooling years). Individuals with missing data on body weight or height, baseline smoking status, leisure-time physical activity or schooling years were excluded from the analyses (n = 4,606, 7.6 %).
Definition of cancer incidence
Information on incidence of cancers was obtained from the Finnish Cancer Registry (FCR) and the dates of deaths from the cause-of-death register of Statistics Finland by computer-based record linkage using the unique personal identity codes assigned to every resident of Finland. The FCR has collected data on all cancer cases diagnosed in Finland since 1953. All physicians, hospitals, and pathology, cytology and hematology laboratories in the country are obligated to send a notification to the FCR of all diagnosed and suspected cancer cases since 1961. In addition, Statistics Finland annually sends to the registry a computerized file of death certificates in which a malignant disease is mentioned. The data coverage in the FCR is virtually complete, 99 % for solid tumors, and the data accuracy is high as previously validated by different researchers [49].
The FCR uses International Classification of Diseases for Oncology, 3rd (ICD-O-3) in classification of the cancer cases. For the current study, cancers were categorised into following sites: any site (C000–C809), stomach (ICD-O-3 topography C16), colon (C18–C19), rectum (C20), liver (C220), gallbladder and extrahepatic bile ducts (C23–C24), pancreas (C25), lung (C34), breast (C50), cervix uteri (C53), ovary (C56), prostate (C61), kidney (C649) and bladder (C67). Only the first occurrence of cancer after the baseline examination was included in the analysis, subsequent cancers of the same site or not were excluded and subjects with a cancer diagnosed before the baseline survey were excluded from the cohort (n = 1,173, 1.9 %). Follow-up of each cohort member started from the date of baseline survey and continued until the date of first cancer diagnosis, date of death, or 31 December, 2008, whichever was the earliest.
Statistical analyses
The Cox proportional hazards model was used to estimate the hazard ratios (HR) and 95 % confidence intervals (CI) of BMI in relation to incidence of cancers, adjusted for baseline smoking status, leisure-time physical activity, education and area using attained age as the time scale. All analyses were performed separately for men and women. The shape of the relationship between BMI and incidence of cancer was explored using both parametric models (conventional linear or polynomial model) and nonparametric models (the linear spline or restricted cubic spline regression model). Akaike’s information criterion (AIC) was used to judge the model fitness between conventional linear model and polynomial models (including quadratic, cubic or fractional polynomial model), the lower the AIC value the better the model fitness is. The reduction of AIC is evaluated by the likelihood ratio test (LRT) or a deviance difference test [50, 51]. We subsequently fitted nonparametric smooth functions, using the restricted cubic spline or the linear spline corresponding to the best-fitting conventional polynomial or linear model [52]. The existence of threshold was estimated by a piecewise regression model using nonlinear least-squares estimation incorporating the “nl” function in Stata, with the lowest value of residual sum of squares, root-mean-square error and AIC, whereas the highest value of R-squared as the criteria for model selection of the optimal threshold or thresholds for BMI. In the Cox proportional hazards model, BMI was entered as a continuous variable, and factors including leisure-time physical activity, smoking status and education as categorical variables, along with a BMI-leisure-time physical activity, a BMI-smoking status or a BMI-education interaction term. In addition, HR for categorical BMI with incidence of cancer was also estimated by the Cox proportional hazards model using BMI category of 23.0–24.9 kg/m2 as the reference category. The proportional hazard assumption of each model was tested for specific variables and globally. The proportional hazards assumptions were met. Data analyses were performed in Stata Intercooled 11.2 (StataCorp, College Station, TX, USA).
Results
During the follow-up (mean 20.6 years), 8,429 (15.4 %) incident cancers were recorded (Table 1), 4,208 from men and 4,221 from women. The mean BMI was higher in older individuals, and persons with a low BMI tended to be more often to smoke (p < 0.05), to be physically active and more educated (p < 0.05 for trend test, Table 2).
Table 3 shows that the best-fitting conventional model was conventional linear model for BMI in relation to incidence of cancers of the colon, liver and kidney in men and the gallbladder and breast in women (all p < 0.05 for LRT against their basic model), as well as the bladder and all sites combined in men and the stomach, colon, lung and ovary in women (all p > 0.05 for LRT against their basic model), and conventional polynomial model did not significantly improve the model fitness (p > 0.05 for LRT or for deviance difference test against their conventional linear model), which suggests a linear relationship. To the contrary, the conventional polynomial model significantly improved the model fitness of incidence of cancers of the lung in men (all p < 0.05 for LRT or deviance difference test of against the conventional linear model), prostate in men as well as all sites combined in women (p < 0.05 for LRT of quadratic polynomial model and p < 0.05 for deviance difference test of second-order fractional polynomial model against their basic model), which suggests a nonlinear relationship. In addition, model fitness for incidence of cancers of other sites was not improved with any term of BMI added (Table 3). HRs with 95 % CIs for incidence of cancer estimated based on the spline regression models were plotted against BMI in Fig. 1. The spline regression analysis showed that BMI had a non-threshold linear positive association with incidence of cancers of the colon, liver, kidney, bladder and all sites combined in men (Fig. 1c, g, s, u and w), and of cancers of the stomach, colon, gallbladder and ovary in women (Fig. 1b, d, j and q). BMI had an inverse association with incidence of cancers of the lung in men (Fig. 1m) and the lung and breast in women (Fig. 1n, o), whereas a J-shaped association with incidence of all cancers combined in women (Fig. 1x), which indicates there might be a threshold for incidence of cancers of the lung in men and the breast in women or two thresholds for incidence of all cancers combined in women existing. No association was observed for BMI and incidence of cancers of other sites (Fig. 1a, e, f, h, i, k, l, p, r, t and v). Threshold estimated for BMI by the non-linear least squares regression analysis based on the spline regression model was shown in Fig. 1 by the vertical lines and in Online Resource 1. Moreover, the relationship between BMI and incidence of cancer was confirmed by the results from analyses using the Cox proportional hazards model as presented as HR (95 % CI) for categorical BMI in Online Resource 2.
Hazard ratio and 95 % confidence intervals for body mass index (BMI) in relation to incidence of cancer among men and women. Dashed lines indicate hazard ratios and 95 % confidence intervals (95 % CIs) derived from the linear spline regression with one interior knot placed at the 50th percentiles of BMI (a–l, n–q and s–w), or from the restricted cubic spline regression with three interior knots placed at the 10th, 50th, and 90th percentiles of BMI (m, r and x), using age as the underlying time-scale in the Cox proportional hazards model adjusted for smoking status, leisure-time physical activity, education and area, and mean of BMI was set as the reference value (figure not shown for certain cancers with BMI > 45 kg/m2 due to their extreme high HRs). Vertical dot line indicates the location which represents threshold (with 95 % CIs denoted in subtitle) derived from the piecewise regression model followed by the spline regression model. Solid lines indicate slopes before and after thresholds
Since the interaction between linear BMI and smoking status was significant for incidence of all cancers combined in women (p = 0.01), an analysis was performed separately for smokers and never smokers. High BMI in women was associated with an increased overall cancer risk in never smokers but a reduced risk in smokers (Online Resource 3). The results were not altered much after excluding the first five years of follow-up (Online Resource 4).
Discussion
BMI had a non-threshold linear positive association with incidence of cancers of the colon, liver, kidney, bladder and all sites combined in men, and of cancers of the stomach, colon, gallbladder and ovary in women, an inverse association with incidence of cancers of the lung in men and the lung and breast in women, a J-shaped association with incidence of all cancers combined in women. In women, high BMI was associated with an increased overall cancer risk in never smokers but a reduced risk in smokers.
BMI was positively associated with incidence of colon cancer, but had no association with incidence of rectal cancer, which is in line with previous studies [2–7, 26–29]. In spite of the fact that liver cancer and gallbladder cancer are relatively rare diseases, we still found BMI had a positive association with incidence of liver cancer in men and gallbladder cancer in women, which is in line with previous studies [3, 6, 30, 31, 33, 34]. Obese patients tend to have non-alcoholic fatty liver disease or gallstone disease, which might mediate the carcinogenesis [34, 53–56]. In line with previous studies [6, 10, 13–15], we found that BMI was positively associated with incidence of kidney cancer in men, which may be due to promoting kidney damage through oxidative stress [57], diabetes or hypertension [58, 59], or altered circulating concentrations of hormones [60, 61].
In addition, a non-significant linear positive relationship was observed between BMI and incidence of cancers of other sites including the bladder in men and the stomach and ovary in women, no association was observed between BMI and incidence of pancreatic cancer and cervical cancer. In recent studies, elevated BMI has been linked with an increased risk of incidence of cancers of the pancreas [3, 8–12] and ovary [10, 37–40], but it is still controversial about the relationship between BMI and incidence of stomach cancer [6, 10, 32], bladder cancer [3, 6, 10, 35, 36] or cervical cancer [3, 10, 33]. Taken together, excess body fat is associated with elevated production of insulin, leading to the increase of insulin-like growth factor I (IGF-I), or secretes a variety of cytokines and sex steroids, which subsequently stimulates cell proliferation and suppresses apoptosis, or chronic inflammation, and thus has been suggested to play a role in the carcinogenesis [62–65]. Elevated BMI was inversely associated with incidence of lung cancer, which is consistent with previous studies [6, 10, 16–18]. Further, no significant interaction was detected between BMI and smoking status, which still needs to be confirmed, for the possible metabolic effects of smoking on body weight [66, 67]. A recent epigenetic study reported that one allele of the fat mass and obesity-associated gene that had been linked with elevated BMI, was associated with a decreased risk of incidence of lung cancer, independent of smoking or weight loss due to the preclinical disease [68].
We found that there was a significant inverse association between BMI and incidence of breast cancer in women. A declining risk association among premenopausal women but an increasing risk association among postmenopausal women has been reported in prvious studies [3, 10, 41, 43–47]. Possible differences in these associations could be related to loss of normal ovarian function with reduced ovarian oestrogens production as the mechanism by which obesity could reduce the tumor promoting effects in premenopausal women, but enhanced oestrogen synthesis by adipose tissue that could contribute to an increased risk of breast cancer among postmenopausal women [69, 70].
Moreover, the possible explanation for the no association of BMI with incidence of prostate cancer observed in our study is that although with lower levels of sex hormone-binding globulin (SHBG) detected [3, 19–25, 45], which is a risk factor for prostate cancer, obese men also possess lower levels of testosterone and IGF-I levels, which may exert a protective effect against the adverse effect of lower levels of SHBG [20]. Furthermore, we did not have information on stage of prostate cancer, since some studies revealed that elevated BMI was associated with an increased risk of incidence of high-graded or aggressive prostate cancer [22, 24, 25], but with a decreased risk of incidence of localized or low-graded prostate cancer [23–25], the mutual coupling effect might be compensated.
We found that in women, high BMI in women was associated with an increased overall cancer risk in never smokers but a reduced risk in smokers, with significant interaction detected between BMI and smoking status. But no significant interaction was detected between BMI and leisure-time physical activity, or between BMI and education in relation to incidence of cancer. Physically active individuals tend to be leaner, physical activity has been reported to attenuate or eliminate the relation between BMI and the risk of incidence of cancers of the colon, rectum and pancreas [5, 8], perhaps through improving insulin resistance and increasing adiponectin levels [71–73]. High educated individuals tend to be leaner, education may have an impact on energy balance by influencing obesity-related behaviours such as diet and physical activity [74, 75].
The strengths of the study are its large cohort size, population-based setting, large number of certain cancer cases accumulated during the follow-up, and completeness of the follow-up due to the well-organized registration system in Finland. But we do not have precise information on weight changes before the baseline and during the follow-up, nor on menopausal status and hormone therapies that would have been potentially affected the development of breast cancer [41, 43, 44, 46, 47]. Because BMI does not accurately differentiate fat mass from muscles, as an endocrine organ, visceral adipose tissue has been suggested to play a more important role in the carcinogenesis than the subcutaneous compartment [63, 64], it may be sometimes misleading to use BMI as an index for obesity in relation to cancer incidence [70, 76]. Thus, investigations on body composition or fat distribution in relation to cancer incidence are needed but the feasibility to conduct such an observational study is low. In addition, we do not have information on several lifestyles or behaviour factors, for instance, dietary factors or alcohol consumption, which might contribute to obesity, and is an independent etiologic factor for several cancers, especially for stomach cancer and liver cancer [33, 77]. Moreover, we have relatively few cases among never smokers and hence cannot rule out the possibility of interactions between smoking and BMI in relation to incidence of lung cancer.
Elevated BMI was associated with an increased risk of incidence of cancers of certain sites. Given that the prevalence of obesity is increasing globally, there is an urgent need to understand the mechanisms linking obesity and cancer, and to develop strategy to prevent cancers.
References
World Health Organisation. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee WHO Technical Report Series. WHO: Geneva; 1995.
Ford ES. Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemiol. 1999;150:390–8.
Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–7.
Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31.
Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42:2590–7.
Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9.
Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL, et al. Body size, weight change, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2978–86.
Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–9.
Patel AV, Rodriguez C, Bernstein L, Chao A, Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of pancreatic cancer in a large US Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:459–66.
Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134–44.
Jiao L, Berrington de Gonzalez A, Hartge P, Pfeiffer RM, Park Y, Freedman DM, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21:1305–14.
Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int J Cancer. 2011;129:1708–17.
Bjorge T, Tretli S, Engeland A. Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. 2004;160:1168–76.
Luo J, Margolis KL, Adami HO, Lopez AM, Lessin L, Ye W, et al. Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: the Women’s Health Initiative (United States). Am J Epidemiol. 2007;166:752–9.
Adams KF, Leitzmann MF, Albanes D, Kipnis V, Moore SC, Schatzkin A, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008;168:268–77.
Kabat GC, Miller AB, Rohan TE. Body mass index and lung cancer risk in women. Epidemiology. 2007;18:607–12.
Kabat GC, Kim M, Hunt JR, Chlebowski RT, Rohan TE. Body mass index and waist circumference in relation to lung cancer risk in the Women’s Health Initiative. Am J Epidemiol. 2008;168:158–69.
Smith L, Brinton LA, Spitz MR, Lam TK, Park Y, Hollenbeck AR, et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst. 2012;104:778–89.
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:557–63.
Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–9.
Lee IM, Sesso HD, Paffenbarger RS Jr. A prospective cohort study of physical activity and body size in relation to prostate cancer risk (United States). Cancer Causes Control. 2001;12:187–93.
MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body size and composition and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:1417–21.
Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–84.
Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:63–9.
Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, et al. Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2008;17:3252–61.
Terry PD, Miller AB, Rohan TE. Obesity and colorectal cancer risk in women. Gut. 2002;51:191–4.
Bowers K, Albanes D, Limburg P, Pietinen P, Taylor PR, Virtamo J, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol. 2006;164:652–64.
Adams KF, Leitzmann MF, Albanes D, Kipnis V, Mouw T, Hollenbeck A, et al. Body mass and colorectal cancer risk in the NIH-AARP cohort. Am J Epidemiol. 2007;166:36–45.
Kuchiba A, Morikawa T, Yamauchi M, Imamura Y, Liao X, Chan AT, et al. Body mass index and risk of colorectal cancer according to fatty acid synthase expression in the nurses’ health study. J Natl Cancer Inst. 2012;104:415–20.
Robsahm TE, Tretli S. Height, weight and gastrointestinal cancer: a follow-up study in Norway. Eur J Cancer Prev. 1999;8:105–13.
Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16:987–96.
Sjodahl K, Jia C, Vatten L, Nilsen T, Hveem K, Lagergren J. Body mass and physical activity and risk of gastric cancer in a population-based cohort study in Norway. Cancer Epidemiol Biomarkers Prev. 2008;17:135–40.
Wolk A, Gridley G, Svensson M, Nyren O, McLaughlin JK, Fraumeni JF, et al. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control. 2001;12:13–21.
Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–8.
Koebnick C, Michaud D, Moore SC, Park Y, Hollenbeck A, Ballard-Barbash R, et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2008;17:1214–21.
Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120:140–6.
Lukanova A, Bjor O, Kaaks R, Lenner P, Lindahl B, Hallmans G, et al. Body mass index and cancer: results from the Northern Sweden Health and Disease Cohort. Int J Cancer. 2006;118:458–66.
Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck AR, et al. Body mass index and risk of ovarian cancer. Cancer. 2009;115:812–22.
Lahmann PH, Cust AE, Friedenreich CM, Schulz M, Lukanova A, Kaaks R, et al. Anthropometric measures and epithelial ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2010;126:2404–15.
World Cancer Research Fund. Ovarian Cancer 2014 Report. Food, Nutrition, Physical Activity, and the Prevention of Ovarian Cancer. http://www.dietandcancerreport.org/cup/cup_resources.php. June 2013.
Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–11.
van den Brandt PA, Dirx MJ, Ronckers CM, van den Hoogen P, Goldbohm RA. Height, weight weight change, and postmenopausal breast cancer risk: the Netherlands Cohort Study. Cancer Causes Control. 1997;8:39–47.
van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–27.
Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13:741–51.
Jonsson F, Wolk A, Pedersen NL, Lichtenstein P, Terry P, Ahlbom A, et al. Obesity and hormone-dependent tumors: cohort and co-twin control studies based on the Swedish Twin Registry. Int J Cancer. 2003;106:594–9.
Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2004;111:762–71.
Anderson GL, Neuhouser ML. Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res (Phila). 2012;5:515–21.
Vartiainen E, Jousilahti P, Alfthan G, Sundvall J, Pietinen P, Puska P. Cardiovascular risk factor changes in Finland, 1972–1997. Int J Epidemiol. 2000;29:49–56.
Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol. 1994;33:365–9.
Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65.
Huelsenbeck JP, Hillis DM, Nielsen R. A likelihood-ratio test of monophyly. Syst Biol. 1996;45:546–58.
Patrick R, Willi S. Multivariable modelling with cubic regression splines: a principled approach. Stata J. 2007;7:45–70.
Wood R, Fraser LA, Brewster DH, Garden OJ. Epidemiology of gallbladder cancer and trends in cholecystectomy rates in Scotland, 1968–1998. Eur J Cancer. 2003;39:2080–6.
Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–19.
Qian Y, Fan JG. Obesity, fatty liver and liver cancer. Hepatobiliary Pancreat Dis Int. 2005;4:173–7.
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–89.
Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension. 2001;37:554–60.
Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci. 2002;324:127–37.
Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005;45:1042–9.
de Jong PE, Verhave JC, Pinto-Sietsma SJ, Hillege HL, PREVEND study group. Obesity and target organ damage: the kidney. Int J Obes Relat Metab Disord. 2002;26(Suppl 4):S21–4.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91.
Jenkins PJ, Bustin SA. Evidence for a link between IGF-I and cancer. Eur J Endocrinol. 2004;151(Suppl 1):S17–22.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56.
Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83.
Osorio-Costa F, Rocha GZ, Dias MM, Carvalheira JB. Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arq Bras Endocrinol Metabol. 2009;53:213–26.
Hodge AM, Westerman RA, de Courten MP, Collier GR, Zimmet PZ, Alberti KG. Is leptin sensitivity the link between smoking cessation and weight gain? Int J Obes Relat Metab Disord. 1997;21:50–3.
Ruige JB, Dekker JM, Blum WF, Stehouwer CD, Nijpels G, Mooy J, et al. Leptin and variables of body adiposity, energy balance, and insulin resistance in a population-based study. The Hoorn Study. Diabetes Care. 1999;22:1097–104.
Brennan P, McKay J, Moore L, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, et al. Obesity and cancer: mendelian randomization approach utilizing the FTO genotype. Int J Epidemiol. 2009;38:971–5.
Rose DP, Vona-Davis L. Interaction between menopausal status and obesity in affecting breast cancer risk. Maturitas. 2010;66:33–8.
Amadou A, Ferrari P, Muwonge R, Moskal A, Biessy C, Romieu I, et al. Overweight, obesity and risk of premenopausal breast cancer according to ethnicity: a systematic review and dose-response meta-analysis. Obes Rev. 2013;14:665–78.
Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–54.
Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–5.
Bluher M, Brennan AM, Kelesidis T, Kratzsch J, Fasshauer M, Kralisch S, et al. Total and high-molecular weight adiponectin in relation to metabolic variables at baseline and in response to an exercise treatment program: comparative evaluation of three assays. Diabetes Care. 2007;30:280–5.
Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K. Educational level, relative body weight, and changes in their association over 10 years: an international perspective from the WHO MONICA Project. Am J Public Health. 2000;90:1260–8.
Rohrmann S, Steinbrecher A, Linseisen J, Hermann S, May A, Luan J, et al. The association of education with long-term weight change in the EPIC-PANACEA cohort. Eur J Clin Nutr. 2012;66:957–63.
Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005.
Gonzalez CA, Sala N, Rokkas T. Gastric cancer: epidemiologic aspects. Helicobacter. 2013;18(Suppl 1):34–8.
Acknowledgments
This study was supported by grants from Academy Finland (1129197, 136895 and 141005), Finnish Cancer Research Foundation and European Foundation for the Study of Diabetes.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Song, X., Pukkala, E., Dyba, T. et al. Body mass index and cancer incidence: the FINRISK study. Eur J Epidemiol 29, 477–487 (2014). https://doi.org/10.1007/s10654-014-9934-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-014-9934-z