Abstract
We analyzed lung cancer incidence among non-smokers, continuing smokers, and ex-smokers in the Nurses Health Study (NHS) and the Health Professionals Follow-Up Study (HPFS) using the two-stage clonal expansion (TSCE) model. Age-specific lung cancer incidence rates among non-smokers are identical in the two cohorts. Within the framework of the model, the main effect of cigarette smoke is on the promotion of partially altered cells on the pathway to cancer. Smoking-related promotion is somewhat higher among women, whereas smoking-related malignant conversion is somewhat lower. In both cohorts the relative risk for a given daily level of smoking is strongly modified by duration. Among smokers, the incidence in NHS relative to that in HPFS depends both on smoking intensity and duration. The age-adjusted risk is somewhat larger in NHS, but not significantly so. After smokers quit, the risk decreases over a period of many years and the temporal pattern of the decline is similar to that reported in other recent studies. Among ex-smokers, the incidence in NHS relative to that in HPFS depends both on previous levels of smoking and on time since quitting. The age-adjusted risk among ex-smokers is somewhat higher in NHS, possibly due to differences in the age-distribution between the two cohorts.




Similar content being viewed by others
Notes
We also tested disjoint models for never and ever smokers. However, these models also lead to similar fits, but with a larger number of parameters.
However, a smoking effect on initiation almost doubling the background rate is still consistent with the data.
A gain of eight log-likelihood points with only one more parameter.
Age-adjusted to the 1990 US total population. Please see Materials and Methods for details.
References
Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB (1993) Are female smokers at higher risk for lung cancer than male smokers? A case–control analysis by histologic type. Am J Epidemiol 138(5):281–293
Zang EA, Wynder EL (1996) Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 88(3–4):183–192
Perneger TV (2001) Sex, smoking, and cancer: a reappraisal. J Natl Cancer Inst 93(21):1600–1602
Bain C, Feskanich D, Speizer FE, Thun M, Hertzmark E, Rosner BA, et al (2004) Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst 96(11):826–834
Blot WJ, McLaughlin JK (2004) Are women more susceptible to lung cancer? J Natl Cancer Inst 96(11):812–813
Patel JD, Bach PB, Kris MG (2004) Lung cancer in US women: a contemporary epidemic. JAMA 291(14):1763–1768
Moolgavkar SH, Venzon DJ (1979) Two-event models for carcinogenesis: incidence curves for childhood and adult tumors. Math Biosci 47(1):55–77
Moolgavkar SH, Knudson AG (1981) Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst 66(6):1037–1052
Hazelton WD, Luebeck EG, Heidenreich WF, Moolgavkar SH (2001) Analysis of a historical cohort of Chinese tin miners with arsenic, radon, cigarette smoke, and pipe smoke exposures using the biologically based two-stage clonal expansion model. Radiat Res 156(1):78–94
Hazelton WD, Clements MS, Moolgavkar SH (2005) Multistage carcinogenesis and lung cancer mortality in three cohorts. Cancer Epidemiol Biomarkers Prev 14(5):1171–1181
Heidenreich WF, Luebeck EG, Moolgavkar SH (1997) Some properties of the hazard function of the two-mutation clonal expansion model. Risk Anal 17(3):391–399
US Decennial Life Tables for 1989–91. vol 1. Hyattsville, Maryland: US Department of Health and Human Services, National Center for Health and Statistics; 1997
Gilks WR, Richardson S, Spiegelhalter DJ (eds) (1996) Markov chain Monte Carlo in practice. Interdisciplinary Statistics. Chapman & Hall, London
Bain C, Feskanich D, Speizer FE, Thun M, Hertzmark E, Rosner BA, et al (2004) Erratum. J Natl Cancer Inst 96(23):1796–1797
Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al (2007) Lung cancer incidence in never smokers. J Clin Oncol 25(5):472–478
Rachet B, Siemiatycki J, Abrahamowicz M, Leffondre K (2004) A flexible modeling approach to estimating the component effects of smoking behavior on lung cancer. J Clin Epidemiol 57(10):1076–1085
Luebeck EG, Heidenreich WF, Hazelton WD, Paretzke HG, Moolgavkar SH (1999) Biologically based analysis of the data for the Colorado uranium miners cohort: age, dose and dose-rate effects. Radiat Res 152(4):339–351
Heidenreich WF, Wellmann J, Jacob P, Wichmann HE (2002) Mechanistic modelling in large case–control studies of lung cancer risk from smoking. Stat Med 21(20):3055–3070
Schollnberger H, Manuguerra M, Bijwaard H, Boshuizen H, Altenburg HP, Rispens SM, et al (2006) Analysis of epidemiological cohort data on smoking effects and lung cancer with a multi-stage cancer model. Carcinogenesis 27(7):1432–1444
Burns DM, Shanks TG, Choi W, Thun MJ, Heath CW, Garfinkel L (1997) The American Cancer Society Cancer Prevention Study I: 12-year followup of 1 million men and women. In: Burns DM, Garfinkel L, Samet JM (eds) Changes in cigarette-related disease risks and their implications for prevention and control. Smoking and Tobacco Control, Monograph 8, NIH Publ No 97–4213, pp 113–304
Shriver SP, Bourdeau HA, Gubish CT, Tirpak DL, Davis AL, Luketich JD, et al (2000) Sex-specific expression of gastrin-releasing peptide receptor: relationship to smoking history and risk of lung cancer. J Natl Cancer Inst 92(1):24–33
Siegfried JM, DeMichele MA, Hunt JD, Davis AG, Vohra KP, Pilewski JM (1997) Expression of mRNA for gastrin-releasing peptide receptor by human bronchial epithelial cells. Association with prolonged tobacco exposure and responsiveness to bombesin-like peptides. Am J Respir Crit Care Med 156(2 Pt 1):358–366
Hauptmann M, Lubin JH, Rosenberg P, Wellmann J, Kreienbrock L (2000) The use of sliding time windows for the exploratory analysis of temporal effects of smoking histories on lung cancer risk. Stat Med 19(16):2185–2194
Leffondre K, Abrahamowicz M, Siemiatycki J, Rachet B (2002) Modeling smoking history: a comparison of different approaches. Am J Epidemiol 156(9):813–823
Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R (2000) Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case–control studies. BMJ 321(7257):323–329
Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al (2003) Variations in lung cancer risk among smokers. J Natl Cancer Inst 95(6):470–478
Acknowledgments
We thank the Cancer Intervention and Surveillance Modeling Network (CISNET) Group, Dr. Anup Dewanji and Dr. Jihyoun Jeon for useful suggestions. We acknowledge support from the NIH grants RO1 CA047658 and UO1 CA97415. Financial support: NIH grants RO1 CA047658 and UO1 CA97415.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
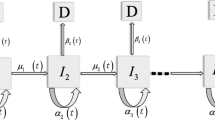
In this section we show how to calculate explicitly the individual likelihoods (Eq. 2) as function of the model parameters and the individual’s smoking history. For simplicity, we assume a constant lag time between the appearance of the first malignant cell and diagnosis, so the survival at any age t, S(t), is equal to the TSCE model survival at age t − t lag , S 2(t − t lag ) (see Eq. 4). We use the expressions derived in Heidenreich et al. [11] for the TSCE model survival in case of piecewise-constant parameters. From Heidenreich et al. [11], we have that the survival function and its derivative at any age t are
where k is the number of age-periods with different smoking-dose before age t k ≡ t − t lag , [t j−1,t j ] denote the end-points of the j − th age-period, d j is the smoking-dose during the j − th age-period, μ0,j , α j , g j , μ1, j denote the parameter values during the j − th age-period and
To calculate the individual likelihood (Eq. 2), we only need to evaluate expressions (5) and (6) at the age at entry (t = a e ) and at the age at last (t = a l ) accordingly.
As an example, we calculate the likelihood contribution of the individual whose smoking history is illustrated in the top panel of Fig. 1 at the maximum likelihood estimates of the joint-model (Table 2). The individual entered the NHS study at age 43 (approx) and died at age 63 from other causes. Hence, we must calculate the TSCE model survival at ages 43 − t lag = 38 and 63 − t lag = 58 to obtain her individual likelihood (Eq. 2). This individual started smoking at age 20, at a constant rate of 30 cigarettes per day, and continued with this pattern until age 42. Hence, to calculate the TSCE model survival at age 38, we have to consider two different age-periods, namely [0, 20]≡[t 0, t 1] and [20, 38]≡[t 1, t 2]. From expression (5), we have that the survival at age 43 is
In this particular example, the parameter values during the first age-period are equal to the background parameters (i.e., d 1 = 0). In contrast, during the second period, we have to multiply the promotion rate g and the malignant conversion rate μ1 by their corresponding dose–responses (with d 2 = 30). We also multiply the initiated cells’ division rate, α, by the dose–response of g in order to keep them consistent. In this model, there is no dose–response on the initiation rate, μ0, so it is constant between all age-periods. Using (Eqs. 5, 7–15) and the parameter estimates from Table 2, we obtain that
So the survival at age 43 is S(43) = S 2(38) = 0.9988. To calculate the TSCE model survival at age 58, we need to consider the following age-periods: \([0, 20]\equiv[t_0,t_1], [20, 42]\equiv[t_1,t_2], [42,44]\equiv[t_2,t_3], [44,46]\equiv[t_3,t_4], [46,48]\equiv[t_4,t_5], [48,55]\equiv[t_5,t_6]\) and [55, 57]≡[t 6, t 7], [57, 58]≡[t 7, t 8]. At each age-period, we must adjust the model parameters by the corresponding dose–response. Similarly as before, we calculate the survival at age 63 using (Eqs. 5, 7–15)
So the likelihood contribution of this individual is equal to
As a final example, let’s assume that an individual with the same smoking history, is diagnosed with lung cancer at age 63. In this case, we must obtain S′(63) = S′2(58) to calculate its individual likelihood (Eq. 2). Using (Eqs. 5–15) we have that
So the likelihood contribution of this individual is equal to
Fortran and R code to compute the TSCE hazard and survival functions in case of piecewise-constant parameters are available from the authors by request.
Rights and permissions
About this article
Cite this article
Meza, R., Hazelton, W.D., Colditz, G.A. et al. Analysis of lung cancer incidence in the nurses’ health and the health professionals’ follow-up studies using a multistage carcinogenesis model. Cancer Causes Control 19, 317–328 (2008). https://doi.org/10.1007/s10552-007-9094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-007-9094-5