Abstract
Purpose
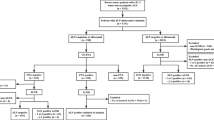
Given the histological special types (HST) of breast carcinoma accounted for minority of the Z0011 study population, this study aimed to assess the rates of axillary lymph node (ALN) involvement and non-sentinel lymph node (SLN) metastasis in patients with invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), or other HST.
Methods
Patients with cT1-2N0M0 breast cancer treated between 2009 and 2018 were retrospectively included from a multi-institutional database. Rates of nodal involvement were analyzed among different histological subgroups. The impact of ALN dissection (ALND) on adjuvant treatment decisions and prognosis were also analyzed among patients with 1–2 + SLNs.
Results
A total of 8294 patients were included: 6854 (82.6%), 257 (3.1%), and 1183 (14.3%) patients with IDC, ILC, and other HST, respectively. IDC patients had a significantly higher rate of ALN metastasis compared with ILC or other HST (31.9% vs. 22.6% vs. 16.4%, P < 0.001). However, in patients with 1–2 + SLNs, rates of non-SLN metastasis were similar among three groups (IDC: n = 182, 28.6% vs. ILC: n = 5, 31.2% vs. other HST: n = 29, 34.9%, P = 0.481). For patients with 1–2 + SLNs, rates of adjuvant chemotherapy and the estimated 3-year recurrence-free survival were similar between the SLN biopsy and ALND arms, regardless of the histological types.
Conclusion
Among patients with 1–2 + SLNs, ILC or other HST had similar rates of non-SLN metastasis compared with IDC. Omission of ALND may not influence adjuvant chemotherapy usage or disease outcome regardless of histological types.




Similar content being viewed by others
Data availability
The datasets analyzed in the present study are available from the corresponding author on reasonable request.
Abbreviations
- ACOSOG:
-
American College of Surgeons Oncology Group
- ALND:
-
Axillary lymph node dissection
- SLN:
-
Sentinel lymph node
- BCS:
-
Breast-conserving surgery
- IDC:
-
Invasive ductal carcinoma
- HST:
-
Histological special type
- ILC:
-
Invasive lobular carcinoma
- Non-SLN:
-
Non-sentinel lymph node
- SJTU-BCDB:
-
Shanghai Jiao Tong University-Breast Cancer Database
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- Her-2:
-
Human epidermal growth factor receptor-2
- SLNR:
-
Sentinel lymph node ratio
- RFS:
-
Recurrence-free survival
- DCIS:
-
Ductal carcinoma in situ
- OS:
-
Overall survival
- SLNB:
-
Sentinel lymph node biopsy
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- ASCO:
-
American Society of Clinical Oncology
References
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6):569–575. https://doi.org/10.1001/jama.2011.90
Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK (2016) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg 264(3):413–420. https://doi.org/10.1097/sla.0000000000001863
Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M (2017) Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 318(10):918–926. https://doi.org/10.1001/jama.2017.11470
Dengel LT, Van Zee KJ, King TA, Stempel M, Cody HS, El-Tamer M, Gemignani ML, Sclafani LM, Sacchini VS, Heerdt AS, Plitas G, Junqueira M, Capko D, Patil S, Morrow M (2014) Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol 21(1):22–27. https://doi.org/10.1245/s10434-013-3200-6
Hennigs A, Köpke M, Feißt M, Riedel F, Rezai M, Nitz U, Moderow M, Golatta M, Sohn C, Schneeweiss A, Heil J (2019) Which patients with sentinel node-positive breast cancer after breast conservation still receive completion axillary lymph node dissection in routine clinical practice? Breast Cancer Res Treat 173(2):429–438. https://doi.org/10.1007/s10549-018-5009-2
Riedel F, Heil J, Feißt M, Rezai M, Moderow M, Sohn C, Schütz F, Golatta M, Hennigs A (2019) Non-sentinel axillary tumor burden applying the ACOSOG Z0011 eligibility criteria to a large routine cohort. Breast Cancer Res Treat 177(2):457–467. https://doi.org/10.1007/s10549-019-05327-4
Cserni G, Bianchi S, Vezzosi V, Arisio R, Bori R, Peterse JL, Sapino A, Drijkoningen M, Kulka J, Eusebi V, Foschini MP, Bellocq J-P, Marin C, Thorstenson S, Amendoeira I, Reiner-Concin A, Decker T (2007) Sentinel lymph node biopsy and non-sentinel node involvement in special type breast carcinomas with a good prognosis. Eur J Cancer 43(9):1407–1414. https://doi.org/10.1016/j.ejca.2007.04.004
Hashmi AA, Aijaz S, Mahboob R, Khan SM, Irfan M, Iftikhar N, Nisar M, Siddiqui M, Edhi MM, Faridi N, Khan A (2018) Clinicopathologic features of invasive metaplastic and micropapillary breast carcinoma: comparison with invasive ductal carcinoma of breast. BMC Res Notes 11(1):531. https://doi.org/10.1186/s13104-018-3623-z
Wang J, Mittendorf EA, Sahin AA, Yi M, Caudle A, Hunt KK, Wu Y (2014) Outcomes of sentinel lymph node dissection alone vs. axillary lymph node dissection in early stage invasive lobular carcinoma: a retrospective study of the surveillance, epidemiology and end results (SEER) database. PLoS ONE 9(2):e89778. https://doi.org/10.1371/journal.pone.0089778
Roberts A, Nofech-Mozes S, Youngson B, McCready DR, Al-Assi M, Ramkumar S, Cil T (2015) The importance of applying ACOSOG Z0011 criteria in the axillary management of invasive lobular carcinoma: a multi-institutional cohort study. Ann Surg Oncol 22(10):3397–3401. https://doi.org/10.1245/s10434-015-4756-0
Adachi Y, Sawaki M, Hattori M, Yoshimura A, Gondo N, Kotani H, Iwase M, Kataoka A, Onishi S, Sugino K, Terada M, Horisawa N, Mori M, Oze I, Iwata H (2018) Comparison of sentinel lymph node biopsy between invasive lobular carcinoma and invasive ductal carcinoma. Breast Cancer 25(5):560–565. https://doi.org/10.1007/s12282-018-0852-x
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Gourgou-Bourgade S, Cameron D, Poortmans P, Asselain B, Azria D, Cardoso F, A'Hern R, Bliss J, Bogaerts J, Bonnefoi H, Brain E, Cardoso MJ, Chibaudel B, Coleman R, Cufer T, Dal Lago L, Dalenc F, De Azambuja E, Debled M, Delaloge S, Filleron T, Gligorov J, Gutowski M, Jacot W, Kirkove C, MacGrogan G, Michiels S, Negreiros I, Offersen BV, Penault Llorca F, Pruneri G, Roche H, Russell NS, Schmitt F, Servent V, Thurlimann B, Untch M, van der Hage JA, van Tienhoven G, Wildiers H, Yarnold J, Bonnetain F, Mathoulin-Pelissier S, Bellera C, Dabakuyo-Yonli TS (2015) Guidelines for time-to-event end point definitions in breast cancer trials: results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials)dagger. Ann Oncol 26(5):873–879. https://doi.org/10.1093/annonc/mdv106
Gebhardt BJ, Thomas J, Horne ZD, Champ CE, Farrugia DJ, Diego E, Ahrendt GM, Beriwal S (2018) Is completion axillary lymph node dissection necessary in patients who are underrepresented in the ACOSOG Z0011 trial? Adv Radiat Oncol 3(3):258–264. https://doi.org/10.1016/j.adro.2018.03.004
Truin W, Roumen RM, Siesling S, van der Heiden-van der Loo M, Lobbezoo DJ, Tjan-Heijnen VC, Voogd AC (2016) Sentinel lymph node biopsy and isolated tumor cells in invasive lobular versus ductal breast cancer. Clin Breast Cancer 16(4):e75–82. https://doi.org/10.1016/j.clbc.2016.03.007
Solà M, Recaj M, Castellà E, Puig P, Gubern JM, Julian JF, Fraile M (2016) Sentinel node biopsy in special histologic types of invasive breast cancer. J Breast Health 12(2):78–82. https://doi.org/10.5152/tjbh.2016.2929
Raber BM, Lin H, Shen Y, Shaitelman SF, Bedrosian I (2019) Trends in regional nodal management of breast cancer patients with low nodal burden. Ann Surg Oncol 26(13):4346–4354. https://doi.org/10.1245/s10434-019-07901-y
Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB III, Bosserman LD, Burstein HJ, Cody H 3rd, Hayman J, Perkins CL, Podoloff DA, Giuliano AE (2014) Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 32:1365–1383. https://doi.org/10.1200/jco.2013.54.1177
Majid S, Rydén L, Manjer J (2019) Determinants for non-sentinel node metastases in primary invasive breast cancer: a population-based cohort study of 602 consecutive patients with sentinel node metastases. BMC Cancer 19(1):626–626. https://doi.org/10.1186/s12885-019-5823-x
Mamtani A, Zabor EC, Stempel M, Morrow M (2019) Lobular histology does not predict the need for axillary dissection among ACOSOG Z0011-eligible breast cancers. Ann Surg Oncol 26(10):3269–3274. https://doi.org/10.1245/s10434-019-07536-z
Corona SP, Bortul M, Scomersi S, Bigal C, Bottin C, Zanconati F, Fox SB, Giudici F, Generali D (2020) Management of the axilla in breast cancer: outcome analysis in a series of ductal versus lobular invasive cancers. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-020-05565-x
Acknowledgements
The authors would like to thank Ms Yidong Du for her work in the development of SJTU-BCDB.
Funding
This study was supported by the National Natural Science Foundation of China (81772797), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20172007), Ruijin Hospital, Shanghai Jiao Tong University School of Medicine—“Guangci Excellent Youth Training Program” (GCQN-2017-A18).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by WG, XF, XC, and YZ. WG, YZ, and XC wrote the first draft of the manuscript, and KS revised the manuscript. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
The present study was reviewed and approved by independent ethical committees of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, W., Zeng, Y., Fei, X. et al. Axillary lymph node and non-sentinel lymph node metastasis among the ACOSOG Z0011 eligible breast cancer patients with invasive ductal, invasive lobular, or other histological special types: a multi-institutional retrospective analysis. Breast Cancer Res Treat 184, 193–202 (2020). https://doi.org/10.1007/s10549-020-05842-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05842-9