Abstract
Background
During the last decade, neoadjuvant chemotherapy (NACT) of early breast cancer (EBC) evolved from a therapy intended to enable operability to a standard treatment option aiming for increasing cure rates equivalent to adjuvant chemotherapy (ACT). In parallel, improvements in the quality control of breast cancer care have been established in specialized breast care units.
Patients and methods
This study analyzed chemotherapy usage in patients with EBC treated at the Heidelberg University Breast Unit between January 2003 and December 2014.
Results
Overall, 5703 patients were included in the analysis of whom 2222 (39 %) received chemotherapy, 817 (37 %) as NACT, and 1405 (63 %) as ACT. The chemotherapy usage declined from 48 % in 2003 to 34 % in 2014 of the cohort. Further, the proportion of NACT raised from 42 to 65 % irrespective of tumor subtype. In addition, frequency of pathologic complete response (pCR) defined as no tumor residues in breast and axilla (ypT0 ypN0) at surgery following NACT increased from 12 % in 2003 to 35 % in 2014. The greatest effect was observed in HER2+ breast cancer with an increase in patients achieving pCR from 24 to 68 %.
Conclusions
The results mirror the refined indication for chemotherapy in EBC and its preferred usage as NACT in Germany. The increase in pCR rate over time suggests improvement in outcome accomplished by a multidisciplinary decision-making process and stringent measures for quality control.
Similar content being viewed by others
Introduction
The insight of early breast cancer (EBC) as a systemic disease was one of the fundamental breakthroughs in breast cancer research and constitutes the basis for our therapy decision making today. As a consequence, adjuvant systemic therapy has become a backbone of EBC treatment. In particular, the evolution of chemotherapy has contributed substantially to the improved outcome of EBC patients today [1]. That includes not only the introduction of innovative cytotoxic drugs such as anthracyclines and taxanes but also the development of novel treatment approaches, i.e., dose-dense regimens and preoperative or neoadjuvant therapy.
Neoadjuvant chemotherapy (NACT) started as a treatment option to enable or improve operability of inflammatory, locally advanced or large breast cancer tumors. After equivalence in survival was confirmed, both adjuvant chemotherapy (ACT) and NACT became the standard treatment options for operable disease [2, 3]. NACT, however, offers a number of unique benefits over ACT. It improves the rate of breast-conserving surgery [2], allows an in vivo testing for drug sensitivity, and provides important prognostic information. The achievement of a pathologic complete response (pCR) defined as no invasive tumor residue in the breast and axilla following NACT is associated with improved disease-free and overall survival with the strongest correlation in aggressive breast cancer subtypes [4, 5]. At the patient level, this has been confirmed recently by a large meta-analysis [6]. Furthermore, post-neoadjuvant treatment may be differentiated depending on whether a patient achieves pCR after NACT or not. This is currently investigated in several trials.
A breast cancer patient’s prognosis also depends on the quality of treatment. To ensure patients are provided with a standard-of-care treatment, the German Cancer Society (DKG) and the German Society for Breast Diseases (DGS) introduced a certification system in 2003. It builds on the sustainable implementation of clinical guidelines in everyday clinical care, the establishment of multidisciplinary teams, and a quality assurance system [7]. The Heidelberg University Breast Care Unit (BCU) was fully certified by the certification board of DKG and DGS in 2003.
We analyzed chemotherapy usage in routine clinical care of a prospective cohort of 5703 patients with primary, non-metastatic breast cancer treated at a specialized BCU between January 2003 and December 2014.
Patient and methods
Patient selection
Data on medical history, demographic characteristics, diagnostics, therapy, and follow-up of all patients referred to the Heidelberg BCU for diagnosis and treatment of primary breast cancer have been prospectively documented since January 1, 2003 in our database. Patients were managed under certified conditions verified continuously by a re-certification process.
Our prospective database comprised 6639 patients with primary breast cancer diagnosed and treated at Heidelberg BCU between January 1, 2003 and December 31, 2014. Nine hundred and thirty-six patients were excluded from the analysis due to male sex (n = 45), no primary diagnosis (n = 23), M1 status at time of diagnosis (n = 364), no surgery, or no histopathological report (n = 504). The selection process is outlined as a Consort diagram in Fig. 1. Patients had provided written informed consent on the use of their demographic and treatment data.
Definitions of tumor histology and stages
Tumor histology was defined according to the World Health Organization criteria [8]; grading was performed and grouped into stages according to the most recent TNM classification [9].
The expression of estrogen receptor (ER), progesterone receptor (PgR), human epidermal growth factor receptor 2 (HER2), and Ki-67 was assessed using formalin-fixed paraffin-embedded tumor tissue according to international standards. Positivity for ER and PgR was defined as an immunoreactive score (IRS) of Remmele and Stegner of ≥1 out of 12 or as a total score (TS) of Allred of ≥1 out of 8. Moreover, any positive staining (i.e., ≥1 %) was defined as positive in accordance with the recent American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline recommendations [10]. HER2 status was defined as positive in case of a semiquantitative HercepTest© score of 3+ by immunohistochemistry (IHC) or in case of a positive FISH/CISH assay as per ASCO/CAP guideline recommendations [11].
Based on ER, PgR, and HER2-status by IHC ± FISH/CISH, three breast cancer subtypes were defined: 1) negative ER, PgR, and HER2 status corresponding to triple negative (TN); 2) positive HER2 status to HER2-positive (HER2+) irrespective of ER and PgR status; and 3) hormone receptor (HR)-positive status defined as a positive ER or a positive PgR status along with a negative HER2 status (HR+ HER2−). For patients with NACT, immunohistochemical information was based on the pre-treatment biopsy, for patients with ACT on the final post-surgery pathological sample.
Statistical analysis
Data were analyzed descriptively using SPSS software version 22 (IBM, Armonk, USA). Annual and biannual percentages of chemotherapy use were calculated and presented as a longitudinal time trend analysis for the period from 2003 to 2014.
Results
Patient and tumor characteristics
The final cohort comprised 5703 patients of which 2222 patients received chemotherapy, 1405 (63 %) as ACT, and 817 (37 %) as NACT (Fig. 1). Demographic and tumor characteristics are presented separately for patients with ACT and NACT (Table 1). In the ACT cohort, the median age of patients was 54 years, more than half of the women were postmenopausal (54 %), and had a tumor grading of G3 in 41 % of the cases. Almost half of the patients had a tumor ≤2 cm (48 %) and were node-negative (48 %).
Patients in the NACT cohort tended to be younger (median age 49 vs. 54 years), had a higher proportion of G3 tumors (53 vs. 41 %), and had more often tumors with a Ki-67 >14 % (78 vs. 65 %) than patients who received ACT. As expected, the tumor size distribution following NACT shows a shift to smaller tumors with a relevant percentage of ypT0 stage (29 %) and lower proportions of (y)pT1 and (y)pT2 tumors (38 vs. 47 % and 19 vs. 41 %, respectively). In the NACT cohort, 58 % of patients had a HR+ HER2−, 16 % a HER2+ , and 26 % a TN subtype as compared to the ACT cohort with 69 % HR+ HER2−, 14 % HER2+, and 17 % TN.
Chemotherapy usage
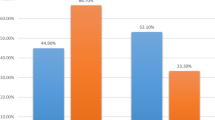
Overall, the annual number of patients diagnosed and treated at our center increased over time, while chemotherapy usage declined. In 2003, 48 % of patients with EBC received chemotherapy compared to only 34 % in 2014 (Fig. 2). This is a steady decrease in the usage of chemotherapy from 2003 to 2014 of 14 %. In parallel, there was a shift in the relative proportions from ACT toward NACT. Figure 3 demonstrates a peak of 84 % for the use of ACT in 2008. The relative proportion of NACT increased continuously thereafter with a steep rise from 2011 to 2012. In 2014, the use of ACT dropped to 35 %, whereas the use of NACT amounted to 65 % (Fig. 3). The shift from ACT to NACT was seen irrespective of breast cancer subtype (Fig. 4). The relative proportion of NACT by breast cancer subtype in 2014 was highest in TN tumors with 75 %, following HER2+ tumors with 70 % and in HR+ HER2− tumors with 58 %.
T-stage distribution
The pT-stage distribution of patients receiving ACT remained stable over the whole period under review (see Supplementary Fig. S1a). However, the relative proportion of ypT-stages in the NACT cohort changed considerably over time. Overall, there was an evolution to more favorable stages after admission of NACT. The percentage of patients with a postoperative ypT0 stage rose continuously from 16 % in 2003/2004 to 37 % in 2013/2014 and the rate of ypTis increased from 3 to 6 %, respectively. At the same time, the relative proportion of ypT1 stages decreased from 48 to 37 % and that of ypT3/4 stages from 16 to 5 %, respectively (see Supplementary Fig. S1b).
Response to neoadjuvant chemotherapy
The shift to more favorable postoperative ypT-stages in the NACT cohort corresponded with an increase in the proportion of patients achieving a pCR over time (Fig. 5). PCR rate according to the most stringent definition (ypT0 ypN0) was 12 % in 2003 rising to 35 % in 2014. The effect was most prominent in tumors of the HER2+ subtype with pCR rising from 24 % in 2003/2004 to 68 % in 2013/2014. An increase in pCR was also seen in HR+ HER2− tumors with a rise from 4 to 23 %, respectively. However, there was no clear trend regarding pCR rates in TN disease (Fig. 6).
pCR (ypT0ypN0) by breast cancer subtype at Heidelberg Breast Care Unit from 2003 to 2014. The graph shows the pCR (ypT0ypN0) among patients receiving neoadjuvant chemotherapy in the three subgroups of immunohistochemically defined breast cancer subtypes at Heidelberg Breast Care Unit from 2003 to 2014
Discussion
Our results reflect the fundamental developments in the usage of chemotherapy in early breast cancer patients. Since the advent of molecular classification systems, it has become evident that systemic therapy of EBC needs to be tailored according to individual risk factors and intrinsic subtype. Modern microarray-based gene expression profiles are the best way to visualize the heterogeneity of breast cancer with a more widely use in clinical routine in recent years. Patients that can be spared chemotherapy have been identified [2, 12]. Thus, it is reassuring that this leads to a substantial decline of overall chemotherapy use in EBC over time.
Another finding of our analysis was the considerable shift from ACT to NACT including a major increase of NACT from 2011 to 2012. To some extent, this may reflect the altered understanding and recognition of NACT. In 2009, the consensus conference of St. Gallen still stated that neoadjuvant systemic therapy was considered justified primarily to enhance the possibility of breast-conserving surgery [13]. However, in 2011, the prognostic value of NACT was finally acknowledged by the St. Gallen consensus conference [12]. NACT use in HER2+ tumors constitutes a significant proportion of the overall NACT use. Thus, the course of the curve may also have been influenced by the availability of trastuzumab outside clinical trials. The peak use of ACT and the minimum use of NACT were in 2007. In 2006, trastuzumab was first approved for adjuvant use in HER2+ EBC, and the application of trastuzumab in HER2+ disease as part of NACT was still deemed investigational [14]. Finally, however at the end of 2011, trastuzumab was also approved in combination with NACT.
While a survey in 2014 of a representative sample of 25 % of all German breast centers found a NACT rate of 15 % in all and of 26 % in highly specialized centers [15], 65 % of EBC patients at our center received NACT in the same period. This high rate is probably grounded on our longstanding engagement as an institution and as individual researchers in studying and developing NACT.
In addition, there was a clear improvement in the response to NACT. Our results compare favorably with the results of the major clinical trials. The pCR rate in all three subtypes documented in 2014 tended to be even higher than what is reported in the literature. While we reached pCR rates (ypT0 ypN0) of 46 % in TN, 68 % in HER2+ and 23 % in HR+ HER2− subtypes, the CTNeoBC pooled analysis reported rates of 34 % for TN, 31 % and 50 % for HER2+ HR+ and HER2+ HR− treated with trastuzumab, and 16 % for HR+ HER2− G3 based on a less conservative pCR definition (ypT0/is ypN0) [6]. The highest pCR (yp T0 ypN0) rates for HER2+ and for HER2+ HR– tumors reported so far were 52 and 84 % with chemotherapy plus trastuzumab and pertuzumab, respectively [16].
It is unlikely that this results from us using a different chemotherapy regimen or a major change in our choice of chemotherapy. In 2003, NACT was already anthracycline and taxane-based [17], and it still is in 2014 [2, 3]. One can argue that in HER2+ disease, the adoption of trastuzumab had an impact. Treatment of HER2+ patients with chemotherapy plus a dual HER2 blockade with trastuzumab and pertuzumab within clinical trials may have contributed to the further improvement from 2011/2012 to 2013/2014. But the improvement in outcomes probably also reflects our learning curve in selecting the appropriate patients with aggressive tumor biology for NACT. We believe they are also the result of the rigorous quality management and control of Heidelberg as a certified BCU. Moreover, it was shown that participation in clinical trials is associated with better outcomes [18].
Finally, achieving pCR is associated with a better prognosis for the individual patient, in particular, for those with aggressive breast cancer subtypes like HR+ G3, HER2+, and TN breast cancer [4–6]. Thus, it is reassuring that we improved in providing this clinical benefit to EBC patients.
References
Cossetti R, Tyldesley SK, Speers CH et al (2015) Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol 33:65–73
AGO Breast Committee, Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer, Guidelines of the AGO Breast Committee, Version 2016.1. http://www.ago-online.de/de/infothek-fuer-aerzte/leitlinienempfehlungen/mamma/. Accessed 27 May 2016
Senkus E, Kyriakides S, Ohno S et al (2015) Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v8–v30
Von Mickwitz G, Untch M, Blohmer JU et al (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796–1804
Houssami N, Macaskill P, von Minckwitz G et al (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 48:3342–3354
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Kowalski C, Ferencz J, Brucker SY et al (2015) Quality of care in breast cancer centers: results of benchmarking by the German Cancer Society and German Society for breast diseases. Breast 24:118–123
Lakhani SR, Ellis I, Schnitt S et al (2012) WHO classification of tumours of the breast, 4th edn. IARC Press, Lyon
Sobin LH, Gospodarowicz MK, Wittekind C (2009) Cancer IUa. TNM classification of malignant tumours, 7th edn. Wiley, New York
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–e72
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22:1736–1747
Goldhirsch A, Ingle JN, Gelber RD et al (2009) Thresholds for therapies: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20:1319–1329
Kaufmann M, Hortobagyi GN, Goldhirsch A et al (2006) Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol 24:1940–1949
Jackisch C, Albert U-S, Bauerfeind I et al. (2014) Die Therapie des frühen Mamma-Karzinoms in Deutschland. http://www.ago-online.de/fileadmin/downloads/leitlinien/mamma/Die_Therapie_des_fr%C3%BChen_Mammakarzinoms_in_Deutschland_2014.pdf. Accessed 9 June 2016
Schneeweiss A, Chia S, Hickish T et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24(9):2278–2284
Von Minckwitz G, Raab G, Caputo A et al (2005) Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 23:2676–2685
Gnant M (2000) Impact of participation in randomized clinical trials on survival of women with early-stage breast cancer—an analysis of 7985 patients. Proc Am Soc Clin Oncol 19:287
Acknowledgments
The authors would like to thank Christian Lange, Brigitte Wiegand, and Ibrahim Kilic for the medical documentation and data management.
Funding
None declared.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interests (e.g., employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, or grants, or other funding with regard to this study) for any of the authors.
Ethics approval and consent to participate
The study was approved by the ethics committee of the University of Heidelberg and in accordance with the Declaration of Helsinki. Because the study was deemed as without risk, including only anonymized analysis of routinely collected data, the ethics committee of the University of Heidelberg did not request approval for consent.
Additional information
J. Heil and A. Schneeweiss authors share senior authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hennigs, A., Riedel, F., Marmé, F. et al. Changes in chemotherapy usage and outcome of early breast cancer patients in the last decade. Breast Cancer Res Treat 160, 491–499 (2016). https://doi.org/10.1007/s10549-016-4016-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4016-4